Omega-3 polyunsaturated fatty acids and its metabolite 12-HEPE rescue busulfan disrupted spermatogenesis via target to GPR120
- PMID: 37743695
- PMCID: PMC10849791
- DOI: 10.1111/cpr.13551
Omega-3 polyunsaturated fatty acids and its metabolite 12-HEPE rescue busulfan disrupted spermatogenesis via target to GPR120
Abstract
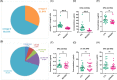
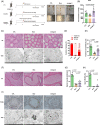
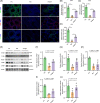
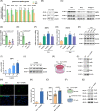
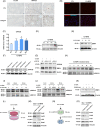
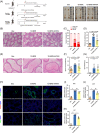
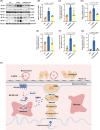
Busulfan is an antineoplastic, which is always accompanied with the abnormal of spermatogonia self-renewal and differentiation. It has been demonstrated that the omega-3 polyunsaturated fatty acids (PUFAs) benefits mature spermatozoa. However, whether omega-3 can protect endogenous spermatogonia and the detailed mechanisms are still unclear. Evaluate of spermatogenesis function (in vivo) were examined by histopathological analysis, immunofluorescence staining, and western blotting. The levels of lipid metabolites in testicular tissue were determined via liquid chromatography. We investigated the effect of lipid metabolites on Sertoli cells provided paracrine factors to regulate spermatogonia proliferation and differentiation using co-culture system. In our study, we showed that omega-3 PUFAs significantly improved the process of sperm production and elevated the quantity of both undifferentiated Lin28+ spermatogonia and differentiated c-kit+ spermatogonia in a mouse model where spermatogenic function was disrupted by busulfan. Mass spectrometry revealed an increase in the levels of several omega-3 metabolites in the testes of mice fed with omega-3 PUFAs. The eicosapentaenoic acid metabolite 12-hydroxyeicosapentaenoic acid (12-HEPE) up-regulated bone morphogenic protein 4 (BMP4) expression through GPR120-ERK1/2 pathway activation in Sertoli cells and restored spermatogonia proliferation and differentiation. Our study provides evidence that omega-3 PUFAs metabolite 12-HEPE effectively protects spermatogonia and reveals that GPR120 might be a tractable pharmacological target for fertility in men received chemotherapy or severe spermatogenesis dysfunction.
© 2023 The Authors. Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Granulocyte colony-stimulating factor (G-CSF) promotes spermatogenic regeneration from surviving spermatogonia after high-dose alkylating chemotherapy.Reprod Biol Endocrinol. 2017 Jan 11;15(1):7. doi: 10.1186/s12958-016-0226-1. Reprod Biol Endocrinol. 2017. PMID: 28077131 Free PMC article.
-
Substance P restores spermatogenesis in busulfan-treated mice: A new strategy for male infertility therapy.Biomed Pharmacother. 2021 Jan;133:110868. doi: 10.1016/j.biopha.2020.110868. Epub 2020 Nov 9. Biomed Pharmacother. 2021. PMID: 33181455
-
Differential distribution of eicosanoids and polyunsaturated fatty acids in the Penaeus monodon male reproductive tract and their effects on total sperm counts.PLoS One. 2022 Sep 22;17(9):e0275134. doi: 10.1371/journal.pone.0275134. eCollection 2022. PLoS One. 2022. PMID: 36137117 Free PMC article.
-
Review: Spermatogenesis in the bull.Animal. 2018 Jun;12(s1):s27-s35. doi: 10.1017/S1751731118000435. Animal. 2018. PMID: 29882505 Review.
-
Age-related presence of spermatogonia in patients with Klinefelter syndrome: a systematic review and meta-analysis.Hum Reprod Update. 2020 Jan 1;26(1):58-72. doi: 10.1093/humupd/dmz038. Hum Reprod Update. 2020. PMID: 31822886
Cited by
-
Ursolic acid attenuates oligospermia in busulfan-induced mice by promoting motor proteins.PeerJ. 2024 Jul 5;12:e17691. doi: 10.7717/peerj.17691. eCollection 2024. PeerJ. 2024. PMID: 38978752 Free PMC article.
-
Multi-omics analysis and experimental verification reveal testicular fatty acid metabolism disorder in non-obstructive azoospermia.Zool Res. 2025 Jan 18;46(1):177-192. doi: 10.24272/j.issn.2095-8137.2024.223. Zool Res. 2025. PMID: 39846195 Free PMC article.
-
[Research progress on glycolipid metabolism of Sertoli cell in the development of spermatogenic cell].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 Mar 25;54(2):257-265. doi: 10.3724/zdxbyxb-2024-0346. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40065698 Free PMC article. Review. Chinese.
-
Octanoic acid mitigates busulfan-induced blood-testis barrier damage by alleviating oxidative stress and autophagy.Lipids Health Dis. 2024 Jun 11;23(1):180. doi: 10.1186/s12944-024-02157-2. Lipids Health Dis. 2024. PMID: 38862993 Free PMC article.
-
Inflammasome activity regulation by PUFA metabolites.Front Immunol. 2024 Sep 3;15:1452749. doi: 10.3389/fimmu.2024.1452749. eCollection 2024. Front Immunol. 2024. PMID: 39290706 Free PMC article. Review.
References
-
- Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31(5):461‐472. - PubMed
-
- Bishop JB, Wassom JS. Toxicological review of busulfan (Myleran). Mutat Res. 1986;168(1):15‐45. - PubMed
-
- de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72(8):580‐585. - PubMed
MeSH terms
Substances
Grants and funding
- SKLRM 2022D3/Independent Research Project of State Key Laboratory of Reproductive Medicine
- JSDW202215/Jiangsu Provincial Medical Key Discipline Cultivation Unit
- 2018YFC1004700/National Key Research and Development Program of China
- 2018YFC1003800/National Key Research and Development Program of China
- U22A20277/National Natural Science Foundation of China
- 81701431/National Natural Science Foundation of China
- 81901547/National Natural Science Foundation of China
- 81971373/National Natural Science Foundation of China
- 81973965/National Natural Science Foundation of China
- 82001535/National Natural Science Foundation of China
- 82001618/National Natural Science Foundation of China
- 82271687/National Natural Science Foundation of China
- KYCX22_1826/Postgraduate Research Practice Innovation Program of Jiangsu Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

