Volatile Organic Compounds Produced by Human Pathogenic Fungi Are Toxic to Drosophila melanogaster
- PMID: 37743879
- PMCID: PMC10512272
- DOI: 10.3389/ffunb.2020.629510
Volatile Organic Compounds Produced by Human Pathogenic Fungi Are Toxic to Drosophila melanogaster
Abstract
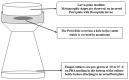
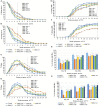
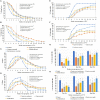
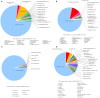
Volatile organic compounds (VOCs) are low molecular mass organic compounds that easily evaporate at room temperature. Fungi produce diverse mixtures of VOCs, some of which may contribute to "sick building syndrome," and which have been shown to be toxigenic in a variety of laboratory bioassays. We hypothesized that VOCs from medically important fungi might be similarly toxigenic and tested strains of Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Cryptococcus gattii, and Saccharomyces cerevisiae in a Drosophila melanogaster eclosion bioassay. Fungi were grown in a shared microhabitat with third instar larvae of D. melanogaster such that there was no physical contact between flies and fungi. As the flies went through metamorphosis, the numbers of larvae, pupae, and adults were counted daily for 15 days. After 8 days, ~80% of controls had eclosed into adults and after 15 days the controls yielded 96-97% eclosion. In contrast, eclosion rates at 8 days were below 70% for flies exposed to VOCs from six different A. fumigatus strains; the eclosion rate at 15 days was only 58% for flies exposed to VOCs from A. fumigatus strain SRRC 1607. When flies were grown in a shared atmosphere with VOCs from S. cerevisiae, after 15 days, 82% of flies had eclosed into adults. Exposure to the VOCs from the medically important yeasts Candida albicans, Cryptococcus neoformans, and Cryptococcus gattii caused significant delays in metamorphosis with eclosion rates of 58% for Candida albicans, 44% for Cryptococcus neoformans, and 56% for Cryptococcus gattii. Using gas chromatography-mass spectrometry, the VOCs from the most toxic and least toxic strains of A. fumigatus were assayed. The two most common VOCs produced by both strains were 1-octen-3-ol and isopentyl alcohol; however, these compounds were produced in 10-fold higher concentrations by the more toxic strain. Our research demonstrates that gas phase compounds emitted by fungal pathogens may have been overlooked as contributing to the pathogenicity of medically important fungi and therefore deserve more scrutiny by the medical mycology research community.
Keywords: Aspergillus fumigatus; Candida albicans; Cryptococcus gattii; Cryptococcus neoformans; Drosophila melanogaster; Virulence; volatile organic compounds.
Copyright © 2021 Almaliki, Angela, Goraya, Yin and Bennett.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

