Biosynthesis of Antibacterial Iron-Chelating Tropolones in Aspergillus nidulans as Response to Glycopeptide-Producing Streptomycetes
- PMID: 37744088
- PMCID: PMC10512232
- DOI: 10.3389/ffunb.2021.777474
Biosynthesis of Antibacterial Iron-Chelating Tropolones in Aspergillus nidulans as Response to Glycopeptide-Producing Streptomycetes
Abstract
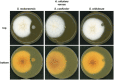
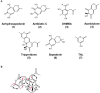
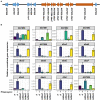
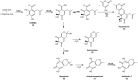
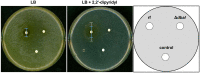
The soil microbiome comprises numerous filamentous fungi and bacteria that mutually react and challenge each other by the production of bioactive secondary metabolites. Herein, we show in liquid co-cultures that the presence of filamentous Streptomycetes producing antifungal glycopeptide antibiotics induces the production of the antibacterial and iron-chelating tropolones anhydrosepedonin (1) and antibiotic C (2) in the mold Aspergillus nidulans. Additionally, the biosynthesis of the related polyketide tripyrnidone (5) was induced, whose novel tricyclic scaffold we elucidated by NMR and HRESIMS data. The corresponding biosynthetic polyketide synthase-encoding gene cluster responsible for the production of these compounds was identified. The tropolones as well as tripyrnidone (5) are produced by genes that belong to the broad reservoir of the fungal genome for the synthesis of different secondary metabolites, which are usually silenced under standard laboratory conditions. These molecules might be part of the bacterium-fungus competition in the complex soil environment, with the bacterial glycopeptide antibiotic as specific environmental trigger for fungal induction of this cluster.
Keywords: Aspergillus nidulans; Streptomyces; fungal-bacterial co-cultivation; glycopeptide antibiotics; structure elucidation; tropolones.
Copyright © 2022 Gerke, Köhler, Wennrich, Große, Shao, Heinrich, Bode, Chen, Surup and Braus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

