The Critical Period After Stroke Study (CPASS) Upper Extremity Treatment Protocol
- PMID: 37744191
- PMCID: PMC10517370
- DOI: 10.1016/j.arrct.2023.100282
The Critical Period After Stroke Study (CPASS) Upper Extremity Treatment Protocol
Abstract
Objective: To present the development of a novel upper extremity (UE) treatment and assess how it was delivered in the Critical Periods After Stroke Study (CPASS), a phase II randomized controlled trial (RCT).
Design: Secondary analysis of data from the RCT.
Setting: Inpatient and outpatient settings the first year after stroke.
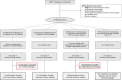
Participants: Of the 72 participants enrolled in CPASS (N=72), 53 were in the study groups eligible to receive the treatment initiated at ≤30 days (acute), 2-3 months (subacute), or ≥6 months (chronic) poststroke. Individuals were 65.1±10.5 years of age, 55% were women, and had mild to moderate UE motor capacity (Action Research Arm Test=17.2±14.3) at baseline.
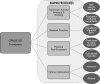
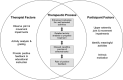
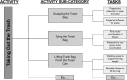
Intervention: The additional 20 hours of treatment began using the Activity Card Sort (ACS), a standardized assessment of activities and participation after stroke, to identify UE treatment goals selected by the participants that were meaningful to them. Treatment activities were broken down into smaller components from a standardized protocol and process that operationalized the treatments essential elements.
Main outcome measures: Feasibility of performing the treatment in a variety of clinical settings in an RCT and contextual factors that influenced adherence.
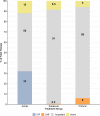
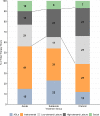
Results: A total of 49/53 participants fully adhered to the CPASS treatment. The duration and location of the treatment sessions and the UE activities practiced during therapy are presented for the total sample (n=49) and per study group as an assessment of feasibility and the contextual factors that influenced adherence.
Conclusions: The CPASS treatment and therapy goals were explicitly based on the meaningful activities identified by the participants using the ACS as a treatment planning tool. This approach provided flexibility to customize UE motor therapy without sacrificing standardization or quantification of the data regardless of the location and UE impairments of participants within the first year poststroke.
Keywords: Clinical Trials as topic; Occupational therapy; Rehabilitation; Stroke rehabilitation; Upper extremity.
Figures
References
-
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Yelnik AP, Quintaine V, Andriantsifanetra C, et al. AMOBES (active mobility very early after stroke): a randomized controlled trial. Stroke. 2017;48:400–405. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

