Biological therapies for premature ovarian insufficiency: what is the evidence?
- PMID: 37744287
- PMCID: PMC10512839
- DOI: 10.3389/frph.2023.1194575
Biological therapies for premature ovarian insufficiency: what is the evidence?
Abstract
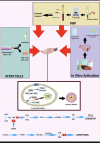
Premature Ovarian Insufficiency (POI) is a multi-factorial disorder that affects women of reproductive age. The condition is characterized by the loss of ovarian function before the age of 40 years and several factors have been identified to be implicated in its pathogenesis. Remarkably though, at least 50% of women have remaining follicles in their ovaries after the development of ovarian insufficiency. Population data show that approximately up to 3.7% of women worldwide suffer from POI and subsequent infertility. Currently, the treatment of POI-related infertility involves oocyte donation. However, many women with POI desire to conceive with their own ova. Therefore, experimental biological therapies, such as Platelet-Rich Plasma (PRP), Exosomes (exos) therapy, In vitro Activation (IVA), Stem Cell therapy, MicroRNAs and Mitochondrial Targeting Therapies are experimental treatment strategies that focus on activating oogenesis and folliculogenesis, by upregulating natural biochemical pathways (neo-folliculogenesis) and improving ovarian microenvironment. This mini-review aims at identifying the main advantages of these approaches and exploring whether they can underpin existing assisted reproductive technologies.
Keywords: HRT; PRP; Premature Ovarian Insufficiency; biological therapies; exosomes; in vitro activation; microRNAs; stem cell therapy.
© 2023 Moustaki, Kontogeorgi, Tsangkalova, Tzoupis, Makrigiannakis, Vryonidou and Kalantaridou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials