Basic CSF parameters and MRZ reaction help in differentiating MOG antibody-associated autoimmune disease versus multiple sclerosis
- PMID: 37744325
- PMCID: PMC10516557
- DOI: 10.3389/fimmu.2023.1237149
Basic CSF parameters and MRZ reaction help in differentiating MOG antibody-associated autoimmune disease versus multiple sclerosis
Abstract
Background: Myelin oligodendrocyte glycoprotein antibody-associated autoimmune disease (MOGAD) is a rare monophasic or relapsing inflammatory demyelinating disease of the central nervous system (CNS) and can mimic multiple sclerosis (MS). The variable availability of live cell-based MOG-antibody assays and difficulties in interpreting low-positive antibody titers can complicate diagnosis. Literature on cerebrospinal fluid (CSF) profiles in MOGAD versus MS, one of the most common differential diagnoses, is scarce. We here analyzed the value of basic CSF parameters to i) distinguish different clinical MOGAD manifestations and ii) differentiate MOGAD from MS.
Methods: This is retrospective, single-center analysis of clinical and laboratory data of 30 adult MOGAD patients and 189 adult patients with relapsing-remitting multiple sclerosis. Basic CSF parameters included CSF white cell count (WCC) and differentiation, CSF/serum albumin ratio (QAlb), intrathecal production of immunoglobulins, CSF-restricted oligoclonal bands (OCB) and MRZ reaction, defined as intrathecal production of IgG reactive against at least 2 of the 3 viruses measles (M), rubella (R) and varicella zoster virus (Z).
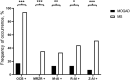
Results: MOGAD patients with myelitis were more likely to have a pleocytosis, a QAlb elevation and a higher WCC than those with optic neuritis, and, after review and combined analysis of our and published cases, they also showed a higher frequency of intrathecal IgM synthesis. Compared to MS, MOGAD patients had significantly more frequently neutrophils in CSF and WCC>30/µl, QAlb>10×10-3, as well as higher mean QAlb values, but significantly less frequently CSF plasma cells and CSF-restricted OCB. A positive MRZ reaction was present in 35.4% of MS patients but absent in all MOGAD patients. Despite these associations, the only CSF parameters with relevant positive likelihood ratios (PLR) indicating MOGAD were QAlb>10×10-3 (PLR 12.60) and absence of CSF-restricted OCB (PLR 14.32), whereas the only relevant negative likelihood ratio (NLR) was absence of positive MRZ reaction (NLR 0.00).
Conclusion: Basic CSF parameters vary considerably in different clinical phenotypes of MOGAD, but QAlb>10×10-3 and absence of CSF-restricted OCB are highly useful to differentiate MOGAD from MS. A positive MRZ reaction is confirmed as the strongest CSF rule-out parameter in MOGAD and could be useful to complement the recently proposed diagnostic criteria.
Keywords: CSF/serum albumin ratio; MOGAD; MRZ reaction; cerebrospinal fluid; multiple sclerosis; oligoclonal bands.
Copyright © 2023 Vlad, Reichen, Neidhart, Hilty, Lekaditi, Heuer, Eisele, Ziegler, Reindl, Lutterotti, Regeniter and Jelcic.
Conflict of interest statement
MR was supported by a research support from Euroimmun and Roche. The University Hospital and Medical University of Innsbruck Austria, employer of Dr. Reindl receives payments for antibody assays MOG, AQP4, and other autoantibodies and for MOG and AQP4 antibody validation experiments organized by Euroimmun Lübeck, Germany. AL received financial compensation and/or travel support for lectures and advice from Biogen, Merck, Novartis, Teva, Genzyme, Roche, Bayer, Celgene and he is a co-founder and co-owner of Cellerys, a company which pursues antigen-specific tolerization. He is co-inventor on a patent held by the University of Zurich on the use of peptide-coupled cells for treatment of MS. IJ has received speaker honoraria or unrestricted grants from Biogen Idec and Novartis and has received compensation for advice or lecturing by for Alexion, Biogen, Bristol Myers Squibb, Celgene, Janssen-Cilag, Neuway, Merck, Novartis, Roche and Sanofi Genzyme; none of these are related to this study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


References
-
- Hoftberger R, Guo Y, Flanagan EP, Lopez-Chiriboga AS, Endmayr V, Hochmeister S, et al. . The pathology of central nervous system inflammatory demyelinating disease accompanying myelin oligodendrocyte glycoprotein autoantibody. Acta Neuropathol (2020) 139:875–92. doi: 10.1007/s00401-020-02132-y - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

