Expression of the ether-a-gò-gò-related gene 1 channel during B and T lymphocyte development: role in BCR and TCR signaling
- PMID: 37744334
- PMCID: PMC10515723
- DOI: 10.3389/fimmu.2023.1111471
Expression of the ether-a-gò-gò-related gene 1 channel during B and T lymphocyte development: role in BCR and TCR signaling
Abstract
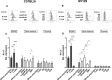
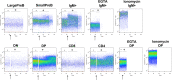
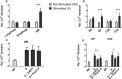
The functional relevance of K+ and Ca2+ ion channels in the "Store Operated Calcium Entry" (SOCE) during B and T lymphocyte activation is well proven. However, their role in the process of T- and B- cell development and selection is still poorly defined. In this scenario, our aim was to characterize the expression of the ether à-go-go-related gene 1 (ERG1) and KV1.3 K+ channels during the early stages of mouse lymphopoiesis and analyze how they affect Ca2+signaling, or other signaling pathways, known to mediate selection and differentiation processes of lymphoid clones. We provide here evidence that the mouse (m)ERG1 is expressed in primary lymphoid organs, bone marrow (BM), and thymus of C57BL/6 and SV129 mice. This expression is particularly evident in the BM during the developmental stages of B cells, before the positive selection (large and small PreB). mERG1 is also expressed in all thymic subsets of both strains, when lymphocyte positive and negative selection occurs. Partially overlapping results were obtained for KV1.3 expression. mERG1 and KV1.3 were expressed at significantly higher levels in B-cell precursors of mice developing an experimental autoimmune encephalomyelitis (EAE). The pharmacological blockage of ERG1 channels with E4031 produced a significant reduction in intracellular Ca2+ after lymphocyte stimulation in the CD4+ and double-positive T-cell precursors' subsets. This suggests that ERG1 might contribute to maintaining the electrochemical gradient responsible for driving Ca2+ entry, during T-cell receptor signaling which sustains lymphocyte selection checkpoints. Such role mirrors that performed by the shaker-type KV1.3 potassium channel during the activation process of mature lymphocytes. No effects on Ca2+ signaling were observed either in B-cell precursors after blocking KV1.3 with PSORA-4. In the BM, the pharmacological blockage of ERG1 channels produced an increase in ERK phosphorylation, suggesting an effect of ERG1 in regulating B-lymphocyte precursor clones' proliferation and checkpoint escape. Overall, our results suggest a novel physiological function of ERG1 in the processes of differentiation and selection of lymphoid precursors, paving the way to further studies aimed at defining the expression and role of ERG1 channels in immune-based pathologies in addition to that during lymphocyte neoplastic transformation.
Keywords: B and T lymphocytes development4; BCR and TCR signalling2; Ca 2+ signalling1; ERG1 K + channel3; ERK phosphorylation; Kv1.3 channel6; SOCE Ca 2+ overload5.
Copyright © 2023 Sala, Staderini, Lottini, Duranti, Angelini, Constantin and Arcangeli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Comment in
-
Editorial: Calcium signaling in cancer immunity.Front Immunol. 2023 Oct 30;14:1315490. doi: 10.3389/fimmu.2023.1315490. eCollection 2023. Front Immunol. 2023. PMID: 38022525 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

