No NLRP3 inflammasome activity in kidney epithelial cells, not even when the NLRP3-A350V Muckle-Wells variant is expressed in podocytes of diabetic mice
- PMID: 37744356
- PMCID: PMC10513077
- DOI: 10.3389/fimmu.2023.1230050
No NLRP3 inflammasome activity in kidney epithelial cells, not even when the NLRP3-A350V Muckle-Wells variant is expressed in podocytes of diabetic mice
Abstract
Background: The NLRP3 inflammasome integrates several danger signals into the activation of innate immunity and inflammation by secreting IL-1β and IL-18. Most published data relate to the NLRP3 inflammasome in immune cells, but some reports claim similar roles in parenchymal, namely epithelial, cells. For example, podocytes, epithelial cells critical for the maintenance of kidney filtration, have been reported to express NLRP3 and to release IL-β in diabetic kidney disease, contributing to filtration barrier dysfunction and kidney injury. We questioned this and hence performed independent verification experiments.
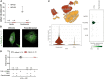
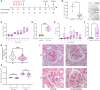
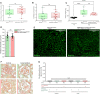
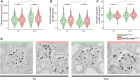
Methods: We studied the expression of inflammasome components in human and mouse kidneys and human podocytes using single-cell transcriptome analysis. Human podocytes were exposed to NLRP3 inflammasome agonists in vitro and we induced diabetes in mice with a podocyte-specific expression of the Muckle-Wells variant of NLRP3, leading to overactivation of the Nlrp3 inflammasome (Nphs2Cre;Nlrp3A350V) versus wildtype controls. Phenotype analysis included deep learning-based glomerular and podocyte morphometry, tissue clearing, and STED microscopy of the glomerular filtration barrier. The Nlrp3 inflammasome was blocked by feeding ß-hydroxy-butyrate.
Results: Single-cell transcriptome analysis did not support relevant NLRP3 expression in parenchymal cells of the kidney. The same applied to primary human podocytes in which NLRP3 agonists did not induce IL-1β or IL-18 secretion. Diabetes induced identical glomerulomegaly in wildtype and Nphs2Cre;Nlrp3A350V mice but hyperfiltration-induced podocyte loss was attenuated and podocytes were larger in Nphs2Cre;Nlrp3A350V mice, an effect reversible with feeding the NLRP3 inflammasome antagonist ß-hydroxy-butyrate. Ultrastructural analysis of the slit diaphragm was genotype-independent hence albuminuria was identical.
Conclusion: Podocytes express low amounts of the NLRP3 inflammasome, if at all, and do not produce IL-1β and IL-18, not even upon introduction of the A350V Muckle-Wells NLRP3 variant and upon induction of podocyte stress. NLRP3-mediated glomerular inflammation is limited to immune cells.
Keywords: IL-1; chronic kidney disease; diabetes; inflammasome; inflammation; proteinuria.
Copyright © 2023 Kunte, Marschner, Klaus, Honda, Li, Motrapu, Walz, Angelotti, Antonelli, Melica, De Chiara, Semeraro, Nelson and Anders.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

