CD4+TGFβ+ cells infiltrated the bursa of Fabricius following IBDV infection, and correlated with a delayed viral clearance, but did not correlate with disease severity, or immunosuppression
- PMID: 37744374
- PMCID: PMC10515216
- DOI: 10.3389/fimmu.2023.1197746
CD4+TGFβ+ cells infiltrated the bursa of Fabricius following IBDV infection, and correlated with a delayed viral clearance, but did not correlate with disease severity, or immunosuppression
Abstract
Introduction: Infectious Bursal Disease Virus (IBDV) causes immunosuppression in chickens. While B-cell destruction is the main cause of humoral immunosuppression, bursal T cells from IBDV-infected birds have been reported to inhibit the mitogenic response of splenocytes, indicating that some T cell subsets in the infected bursa have immunomodulatory activities. CD4+CD25+TGFβ+ cells have been recently described in chickens that have immunoregulatory properties and play a role in the pathogenesis of Marek's Disease Virus.
Methods: To evaluate if CD4+CD25+TGFβ+ cells infiltrated the bursa of Fabricius (BF) following IBDV infection, and influenced the outcome of infection, birds were inoculated at either 2 days or 2 weeks of age with vaccine strain (228E), classic field strain (F52/70), or PBS (mock), and bursal cell populations were quantified by flow cytometry.
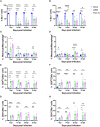
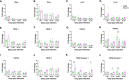
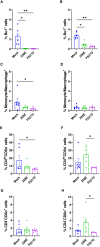
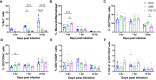
Results: Both 228E and F52/70 led to atrophy of the BF, a significant reduction of Bu1+-B cells, and a significant increase in CD4+ and CD8α+ T cells in the BF, but only F52/70 caused suppression of immune responses to a test antigen in younger birds, and clinical signs in older birds. Virus was cleared from the BF more rapidly in younger birds than older birds. An infiltration of CD4+CD25+T cells into the BF, and elevated expression of bursal TGFβ-1+ mRNA was observed at all time points following infection, irrespective of the strain or age of the birds, but CD4+TGFβ+cells and CD4+CD25+TGFβ+ cells only appeared in the BF at 28 dpi in younger birds. In older birds, CD4+TGFβ+ cells and CD4+CD25+TGFβ+ cells were present at earlier time points, from 7dpi following 228E infection, and from 14 and 28 dpi following F52/70 infection, respectively.
Discussion: Our data suggest that an earlier infiltration of CD4+TGFβ+ cells into the BF correlated with a delayed clearance of virus. However, the influx of CD4+TGFβ+ cells and CD4+CD25+TGFβ+ into the BF did not correlate with increased pathogenicity, or immunosuppression.
Keywords: CD4+CD25+ T cells; IBDV; Infectious bursal disease virus; TGFβ; bursa of Fabricius; immunosuppression; regulatory T cells.
Copyright © 2023 Nazki, Reddy, Kamble, Sadeyen, Iqbal, Behboudi, Shelton and Broadbent.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Eterradossi N, Saif YM. Infectious bursal disease. Dis Poultry. (2020) 257–83. doi: 10.1002/9781119371199.ch7 - DOI
-
- Cazaban C, Gardin Y, Oort R. Gumboro disease-a persisting problem. Libourne: Ceva Santé Animale (2017), 4–5.
Publication types
MeSH terms
Substances
Grants and funding
- BB/T008806/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007030/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007031/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007032/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007035/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

