Hepatic Safety of Febuxostat and Allopurinol for Gout Patients: A Systematic Review of Randomized Controlled Trial
- PMID: 37744559
- PMCID: PMC10516211
- DOI: 10.2147/TCRM.S424598
Hepatic Safety of Febuxostat and Allopurinol for Gout Patients: A Systematic Review of Randomized Controlled Trial
Abstract
Purpose: This study aims to systematically review the hepatic safety of febuxostat and allopurinol in adult gout patients.
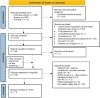
Methods: We searched for information using the following databases: PubMed, Cochrane Library, and Scopus. The inclusion criteria were to review all randomized controlled trials (RCT) that compared allopurinol and febuxostat for adult gout patients that had an assessment of liver function outcomes. Non-English studies on case reports, case series, reviews, and abstracts only were excluded. We extracted information from the studies to answer the research question, ie, study design, publication year, population, sample size, patient characterization, duration, Jadad score, and liver function outcomes.
Results: We screened 512 publications from the databases and identified 11 studies that met the inclusion criteria. Ten out of 11 included studies were double-blind RCTs. In the majority of the included studies, no statistically significant differences were observed in terms of hepatic safety data between febuxostat and allopurinol. However, in studies where allopurinol titration was used, it posed a challenge to maintain blinding. Notably, consistent adverse events related to liver function findings were observed across all reviewed RCTs. These abnormal liver function test results sometimes led to study withdrawal based on the investigators' assessment. Nevertheless, the investigators classified most liver function test elevations as mild to moderate in severity.
Conclusion: Our analysis concluded that adult gout patients enrolled in the included RCTs exhibited similar hepatic safety profiles for both febuxostat and allopurinol treatment. Liver function abnormalities were identified in all RCTs included in this systematic review. Consequently, it is important for the product labeling information of both allopurinol and febuxostat to present and describe the current safety data to guide healthcare practitioners when prescribing these medications to patients. Pharmacovigilance and post-marketing pharmacoepidemiology data are essential in establishing the comprehensive safety profile.
Keywords: allopurinol; febuxostat; gout; hepatic safety.
© 2023 Dewi et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Becker MA, Schumacher HR, Wortmann RL, et al. Febuxostat Compared with Allopurinol in Patients with Hyperuricemia and Gout. Vol 23; 2005. Available from: www.nejm.org. Accessed September 8, 2023. - PubMed
-
- Suzuki S, Yoshihisa A, Yokokawa T, et al. Comparison between febuxostat and allopurinol uric acid-lowering therapy in patients with chronic heart failure and hyperuricemia: a multicenter randomized controlled trial. J Int Med Res. 2021;49(12):030006052110627. doi: 10.1177/03000605211062770 - DOI - PMC - PubMed
-
- Hainer BL, Matheson E, Wilkes RT Diagnosis, Treatment, and Prevention of Gout. Vol 90; 2014. Available from: www.aafp.org/afp. Accessed September 8, 2023. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous