Use of Noncanonical Tyrosine Analogues to Probe Control of Radical Intermediates during Endoperoxide Installation by Verruculogen Synthase (FtmOx1)
- PMID: 37744570
- PMCID: PMC10516331
- DOI: 10.1021/acscatal.2c01037
Use of Noncanonical Tyrosine Analogues to Probe Control of Radical Intermediates during Endoperoxide Installation by Verruculogen Synthase (FtmOx1)
Abstract
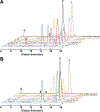
Important bioactive natural products, including prostaglandin H2 and artemisinin, contain reactive endoperoxides. Known enzymatic pathways for endoperoxide installation require multiple hydrogen-atom transfers (HATs). For example, iron(II)- and 2-oxoglutarate-dependent verruculogen synthase (FtmOx1; EC 1.14.11.38) mediates HAT from aliphatic C21 of fumitremorgin B, capture of O2 by the C21 radical (C21•), addition of the peroxyl radical (C21-O-O•) to olefinic C27, and HAT to the resultant C26•. Recent studies proposed conflicting roles for FtmOx1 tyrosine residues, Tyr224 and Tyr68, in the HATs from C21 and to C26•. Here, analysis of variant proteins bearing a ring-halogenated tyrosine or (amino)phenylalanine in place of either residue establishes that Tyr68 is the hydrogen donor to C26•, while Tyr224 has no essential role. The radicals that accumulate rapidly in FtmOx1 variants bearing a HAT-competent tyrosine analog at position 68 exhibit hypsochromically shifted absorption and, in cases of fluorine substitution, 19F-coupled electron-paramagnetic-resonance (EPR) spectra. By contrast, functional Tyr224-substituted variants generate radicals with unaltered light-absorption and EPR signatures as they produce verruculogen. The alternative major product of the Tyr68Phe variant, which forms competitively with verruculogen also in wild-type FtmOx1 in 2H2O and in the variant with the less readily oxidized 2,3-F2Tyr at position 68, is identified by mass spectrometry and isotopic labeling as the 26-hydroxy-21,27-endoperoxide compound formed after capture of another equivalent of O2 by the longer lived C26•. The results highlight the considerable chemical challenges the enzyme must navigate in averting both oxygen rebound and a second O2 coupling to obtain verruculogen selectively over other possible products.
Keywords: 2-oxoglutarate; enzyme mechanism; ferryl; fumitremorgin B endoperoxidase; hydrogen atom transfer; iron; oxygenase; tyrosyl radical.
Figures

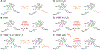



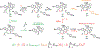
References
-
- Liu D-Z; Liu J-K Peroxy natural products. Nat. Prod. Bioprospect 2013, 3, 161–206.
-
- Wu L; Wang Z; Cen Y; Wang B; Zhou J Structural insight into the catalytic mechanism of endoperoxide synthase FtmOx1. Angew. Chem Int. Ed. 2022, 61, e202112063. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
