Long-term safety and effectiveness of azathioprine in the management of inflammatory bowel disease: A real-world experience
- PMID: 37744710
- PMCID: PMC10517446
- DOI: 10.1002/jgh3.12955
Long-term safety and effectiveness of azathioprine in the management of inflammatory bowel disease: A real-world experience
Abstract
Background and aim: Azathioprine (AZA) forms the cornerstone for maintenance of sustained remission in inflammatory bowel disease (IBD). There is apprehension regarding the long-term effectiveness and safety of AZA in IBD. We present our experience with AZA use and outcomes in a cohort of IBD patients followed up over a long period of time.
Methods: Records of 507 IBD patients under treatment at a single, tertiary care center in south India between 2013 and 2022 were evaluated retrospectively. Long-term compliance, tolerance, clinical outcome at the point of last follow-up, type and duration to the onset of adverse events, and subsequent amendment to treatment with regard to AZA were analyzed.
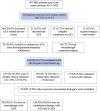
Results: Of 507 patients with IBD, 320 patients (207 Crohn's disease [CD], 113 ulcerative colitis [UC]) who received AZA were included. The median follow-up was 41 months (interquartile range 15.5-77.5). Total duration of exposure was 1359 patient-years with median usage of 33 months. Of the patients, 26.9% received AZA for >5 years. Mean initiation and maximum doses of AZA were 0.97 and 1.72 mg/kg/day. Among the participants, 20.6% experienced side effects, including myelotoxicity (7.2%) and gastrointestinal intolerance (5.6%). Six patients developed malignancy. Among the side effects, 39.4% of side effects were dose-dependent. Among the patients, 38.1% had relapses requiring pulse corticosteroid therapy, and 16.2% had more than one relapse after commencement of AZA. AZA was continued till the last follow-up in 76.5%. Among the patients, 49.7% (UC 51.3, CD 48.8) attained durable remission without biologics, and 5.3% continued to have active disease.
Conclusion: AZA is safe and effective in the long-term in IBD. Effectiveness, tolerance, and compliance with AZA are well sustained beyond 5 years of usage and comparable between UC and CD.
Keywords: adverse events; azathioprine; inflammatory bowel disease; long‐term effectiveness; tolerance.
© 2023 The Authors. JGH Open published by Journal of Gastroenterology and Hepatology Foundation and John Wiley & Sons Australia, Ltd.
Figures
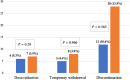
 ), Ulcerative colitis; (
), Ulcerative colitis; ( ), Crohn's disease.
), Crohn's disease.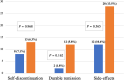
 ), Ulcerative colitis; (
), Ulcerative colitis; ( ), Crohn's disease.
), Crohn's disease.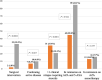
 ), Ulcerative colitis; (
), Ulcerative colitis; ( ), Crohn's disease. ASA, aminosalicylates.
), Crohn's disease. ASA, aminosalicylates.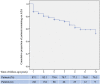
Similar articles
-
Generalized Pyoderma Gangrenosum Associated with Ulcerative Colitis: Successful Treatment with Infliximab and Azathioprine.Acta Dermatovenerol Croat. 2016 Apr;24(1):83-5. Acta Dermatovenerol Croat. 2016. PMID: 27149138
-
Azathioprine is effective in corticosteroid-dependent Asian inflammatory bowel disease patients.Inflamm Bowel Dis. 2011 Mar;17(3):809-15. doi: 10.1002/ibd.21382. Inflamm Bowel Dis. 2011. PMID: 20645318
-
Azathioprine: state of the art in inflammatory bowel disease.Scand J Gastroenterol Suppl. 1998;225:92-9. doi: 10.1080/003655298750027290. Scand J Gastroenterol Suppl. 1998. PMID: 9515759 Review.
-
Comparative effectiveness of azathioprine in Crohn's disease and ulcerative colitis: prospective, long-term, follow-up study of 394 patients.Aliment Pharmacol Ther. 2008 Jul;28(2):228-38. doi: 10.1111/j.1365-2036.2008.03732.x. Epub 2008 May 12. Aliment Pharmacol Ther. 2008. PMID: 18485129
-
Azathioprine: an update on clinical efficacy and safety in inflammatory bowel disease.Scand J Gastroenterol Suppl. 1999;230:111-5. doi: 10.1080/003655299750025633. Scand J Gastroenterol Suppl. 1999. PMID: 10499471 Review.
Cited by
-
Real-World Experience With Tofacitinib in Steroid-Dependent Patients With Moderate-to-Severe Ulcerative Colitis on Immunomodulators.Cureus. 2025 Feb 22;17(2):e79456. doi: 10.7759/cureus.79456. eCollection 2025 Feb. Cureus. 2025. PMID: 40130137 Free PMC article.
-
Laboratory Tests in Inflammatory Bowel Disease: An Evidence-Based Approach to Daily Practice.Biomedicines. 2025 Feb 17;13(2):491. doi: 10.3390/biomedicines13020491. Biomedicines. 2025. PMID: 40002904 Free PMC article. Review.
-
Effects of acupuncture and moxibustion on ulcerative colitis: An overview of systematic reviews.Heliyon. 2024 Mar 8;10(6):e27524. doi: 10.1016/j.heliyon.2024.e27524. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38510004 Free PMC article.
-
Treatment of rheumatoid arthritis-associated interstitial lung disease: An appraisal of the 2023 ACR/CHEST guideline.Curr Treatm Opt Rheumatol. 2024 Dec;10(4):43-60. doi: 10.1007/s40674-024-00217-3. Epub 2024 Sep 16. Curr Treatm Opt Rheumatol. 2024. PMID: 39822854
-
Contemporary and prospective use of azathioprine (AZA) in viral, rheumatic, and dermatological disorders: a review of pharmacogenomic and nanotechnology applications.Naunyn Schmiedebergs Arch Pharmacol. 2025 Apr;398(4):3183-3197. doi: 10.1007/s00210-024-03569-8. Epub 2024 Nov 4. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39495265 Review.
References
-
- Sandborn WJ. Azathioprine: state of the art in inflammatory bowel disease. Scand. J. Gastroenterol. Suppl. 1998; 225: 92–99. - PubMed
-
- Derijks LJ, Gilissen LP, Hooymans PM, Hommes DW. Review article: thiopurines in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006; 24: 715–729. - PubMed
-
- Dubinsky MC, Lamothe S, Yang HY et al. Pharmacogenomics and metabolite measurement for 6‐mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000; 118: 705–713. - PubMed
-
- Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6‐thioguanine nucleotide levels and inflammatory bowel disease activity: a meta‐analysis. Gastroenterology. 2006; 130: 1047–1053. - PubMed
LinkOut - more resources
Full Text Sources

