-Pharmacokinetics of antimalarial drugs used to treat uncomplicated malaria in breastfeeding mother-infant pairs: An observational pharmacokinetic study
- PMID: 37744730
- PMCID: PMC10514676
- DOI: 10.12688/wellcomeopenres.18512.2
-Pharmacokinetics of antimalarial drugs used to treat uncomplicated malaria in breastfeeding mother-infant pairs: An observational pharmacokinetic study
Abstract
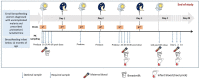
Background: Data surrounding the exposure of the breastfed infant to drugs and any associated risks are sparse. Drugs usually are transferred to milk in small quantities, and many have been used without obviously noticeable infant toxicity for many years - this lack of a 'safety signal' has further reduced the interest in studying mother-to-infant transfer of the drugs. In sub-Saharan Africa, pregnant women are at risk of Plasmodium falciparum infection, and one in four women have evidence of placental infection at the time of delivery. Artemisinin-based combination therapies (ACTs), primarily artemether-lumefantrine (AL), are the current first-line treatment for uncomplicated Plasmodium falciparum malaria, with the same dosing recommendations in breastfeeding women as those in the adult population. Dihydroartemisinin-piperaquine (DP) is routinely used as an alternative to AL in Uganda. However, lactation pharmacokinetics (PK) of ACTs are unknown. Pharmacokinetic characterization of anti-malarial transfer to breast milk and breastfed infants is crucial in understanding the potential consequences to the infant, in terms of therapeutic- and prophylactic effects as well as potential toxicity. Methods: This observational study will enroll 30 mother-infant pairs, and aims to characterize the breastmilk transfer of antimalarial medications (AL and DP) to infants when these ACTs are administered to mothers as part of treatment for uncomplicated malaria. In addition, we will assess the mental health of the breastfeeding mothers enrolled as well as the well-being of their children. PK samples of maternal blood, breastmilk and breastfeeding infant's blood will be obtained at specific times points. Pharmacokinetic data will be analyzed using a population pharmacokinetic approach. Conclusions: We anticipate that findings from this research will guide to develop a PK model describing lumefantrine and piperaquine disposition and will provide a framework to foster other lactation pharmacokinetic studies in different disease areas.
Keywords: Africa; breastmilk; lactation; malaria; pharmacokinetics.
Copyright: © 2023 Nakijoba R et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
Similar articles
-
Pharmacokinetics of drugs used to treat drug sensitive-tuberculosis in breastfeeding mother-infant pairs: An observational pharmacokinetic study.Wellcome Open Res. 2024 Aug 12;8:216. doi: 10.12688/wellcomeopenres.19113.4. eCollection 2023. Wellcome Open Res. 2024. PMID: 38966399 Free PMC article.
-
Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria.Cochrane Database Syst Rev. 2013 Oct 25;2013(10):CD008492. doi: 10.1002/14651858.CD008492.pub3. Cochrane Database Syst Rev. 2013. PMID: 24163021 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
Cited by
-
Pharmacokinetics of drugs used to treat drug sensitive-tuberculosis in breastfeeding mother-infant pairs: An observational pharmacokinetic study.Wellcome Open Res. 2024 Aug 12;8:216. doi: 10.12688/wellcomeopenres.19113.4. eCollection 2023. Wellcome Open Res. 2024. PMID: 38966399 Free PMC article.
-
PBPK-Led Assessment of Antimalarial Drug Concentrations in Breastmilk: A Strategy for Optimal Use of Prediction Methods to Guide Decision Making in an Understudied Population.CPT Pharmacometrics Syst Pharmacol. 2025 Apr;14(4):738-750. doi: 10.1002/psp4.13311. Epub 2025 Feb 11. CPT Pharmacometrics Syst Pharmacol. 2025. PMID: 39930940 Free PMC article.
-
Clinical lactation studies. Acting on key recommendations over the last decade.NPJ Womens Health. 2025;3(1):19. doi: 10.1038/s44294-025-00064-0. Epub 2025 Feb 28. NPJ Womens Health. 2025. PMID: 40028395 Free PMC article. Review.
-
Evaluation of Violacein Metabolic Stability and Metabolite Identification in Human, Mouse, and Rat Liver Microsomes.Pharmaceutics. 2025 May 2;17(5):601. doi: 10.3390/pharmaceutics17050601. Pharmaceutics. 2025. PMID: 40430892 Free PMC article.
-
Pharmacokinetic Research in Pregnancy: Ethical Low-Hanging Fruit?J Clin Pharmacol. 2023 Jun;63 Suppl 1(Suppl 1):S18-S20. doi: 10.1002/jcph.2228. J Clin Pharmacol. 2023. PMID: 37317493 Free PMC article. No abstract available.
References
-
- FDA: Clinical Lactation Studies: Considerations for Study Design. Guidance for Industry.US Department for Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER); 2019. Reference Source
Grants and funding
LinkOut - more resources
Full Text Sources