Cyclodextrin Host-Guest Recognition in Glucose-Monitoring Sensors
- PMID: 37744789
- PMCID: PMC10515351
- DOI: 10.1021/acsomega.3c03746
Cyclodextrin Host-Guest Recognition in Glucose-Monitoring Sensors
Abstract
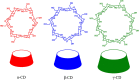
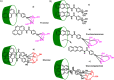
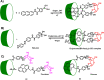
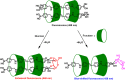
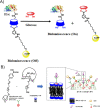
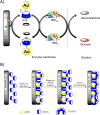
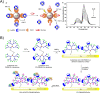
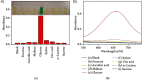
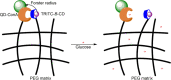
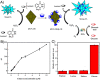
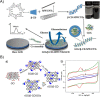
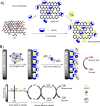
Diabetes mellitus is a prevalent chronic health condition that has caused millions of deaths worldwide. Monitoring blood glucose levels is crucial in diabetes management, aiding in clinical decision making and reducing the incidence of hypoglycemic episodes, thereby decreasing morbidity and mortality rates. Despite advancements in glucose monitoring (GM), the development of noninvasive, rapid, accurate, sensitive, selective, and stable systems for continuous monitoring remains a challenge. Addressing these challenges is critical to improving the clinical utility of GM technologies in diabetes management. In this concept, cyclodextrins (CDs) can be instrumental in the development of GM systems due to their high supramolecular recognition capabilities based on the host-guest interaction. The introduction of CDs into GM systems not only impacts the sensitivity, selectivity, and detection limit of the monitoring process but also improves biocompatibility and stability. These findings motivated the current review to provide a comprehensive summary of CD-based blood glucose sensors and their chemistry of glucose detection, efficiency, and accuracy. We categorize CD-based sensors into four groups based on their modification strategies, including CD-modified boronic acid, CD-modified mediators, CD-modified nanoparticles, and CD-modified functionalized polymers. These findings shed light on the potential of CD-based sensors as a promising tool for continuous GM in diabetes mellitus management.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Guo X.; Li H.; Xu H.; Woo S.; Dong H.; Lu F.; Lange A. J.; Wu C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin B 2012, 2 (4), 358–367. 10.1016/j.apsb.2012.06.002. - DOI
-
- Ozougwu J.; Obimba K.; Belonwu C.; Unakalamba C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J. Physiol Pathophysiol 2013, 4 (4), 46–57. 10.5897/JPAP2013.0001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

