Synthesis and Characterization of Carbon Microbeads
- PMID: 37744801
- PMCID: PMC10515371
- DOI: 10.1021/acsomega.3c05042
Synthesis and Characterization of Carbon Microbeads
Abstract
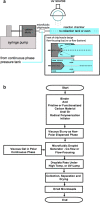
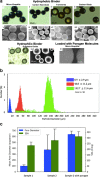
We report a microfluidic-based droplet generation platform for synthesizing micron-sized porous carbon microspheres. The setup employs carbon materials such as graphite, carbon nanotubes, graphene, fullerenes, and carbon black as starting materials. Custom composition, structure, and function are achieved through combinations of carbon materials, cross-linkers, and additives along with variations in process parameters. Carbon materials can be assembled into spheres with a mean diameter of units to hundreds of μm with relatively tight size distribution (<25% RSD). Pore structure and size (tens to hundreds of angstrom) can be modulated by incorporating porogen/coporogen dilutants during synthesis. The microbeads have excellent mechanical stability with an elastic modulus of hundreds of MPa. They can sustain high dynamic fluid flow pressures of up to 9000 psi. This work lays the foundation for synthesizing novel tailorable and customizable carbon microbeads. It opens avenues for applying these novel materials for composite and additive manufacturing, energy, life science, and biomedical applications.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Millennial Scientific and the investigators have filed patents. They are developing commercial products related to the technology reported in this article.
Figures


Similar articles
-
Microfluidic electrospray generation of porous magnetic Janus reduced graphene oxide/carbon composite microspheres for versatile adsorption.J Colloid Interface Sci. 2022 Oct 15;624:546-554. doi: 10.1016/j.jcis.2022.05.156. Epub 2022 Jun 1. J Colloid Interface Sci. 2022. PMID: 35679642
-
A novel and facile synthesis approach for a porous carbon/graphene composite for high-performance supercapacitors.Nanotechnology. 2018 Mar 2;29(9):095401. doi: 10.1088/1361-6528/aaa529. Nanotechnology. 2018. PMID: 29300179
-
Quantitative Coassembly for Precise Synthesis of Mesoporous Nanospheres with Pore Structure-Dependent Catalytic Performance.Adv Mater. 2021 Oct;33(43):e2103130. doi: 10.1002/adma.202103130. Epub 2021 Sep 12. Adv Mater. 2021. PMID: 34510574
-
Synthesis and applications of carbon nanomaterials for energy generation and storage.Beilstein J Nanotechnol. 2016 Feb 1;7:149-96. doi: 10.3762/bjnano.7.17. eCollection 2016. Beilstein J Nanotechnol. 2016. PMID: 26925363 Free PMC article. Review.
-
Advances in preparation, mechanism and applications of various carbon materials in environmental applications: A review.Chemosphere. 2022 Aug;300:134596. doi: 10.1016/j.chemosphere.2022.134596. Epub 2022 Apr 15. Chemosphere. 2022. PMID: 35436457 Review.
Cited by
-
Nanostructured Microsphere Production by Osmotic Extraction of Microfluidic Emulsion Templates.Langmuir. 2025 Aug 19;41(32):21780-21789. doi: 10.1021/acs.langmuir.5c02866. Epub 2025 Aug 6. Langmuir. 2025. PMID: 40769203 Free PMC article.
-
Liquid Chromatography Evaluation of a Novel Graphitic Carbon Stationary Phase Using Glucagon-Like Peptide‑1 Analogs.ACS Omega. 2025 Aug 6;10(32):35666-35677. doi: 10.1021/acsomega.5c02172. eCollection 2025 Aug 19. ACS Omega. 2025. PMID: 40852274 Free PMC article.
References
-
- Deng X.; Li J.; Ma L.; Sha J.; Zhao N. Three-dimensional porous carbon materials and their composites as electrodes for electrochemical energy storage systems. Mater. Chem. Front. 2019, 3 (11), 2221–2245. 10.1039/C9QM00425D. - DOI
-
- Knox J. H.; Gilbert M. T.. Preparation of Porous Carbon. U.S. Patent US4,263,268A, 1981.
-
- Lü Y.; Ling L.; Wu D.; Liu L.; Zhang B.; Mochida I. Preparation of mesocarbon microbeads from coal tar. J. Mater. Sci. 1999, 34 (16), 4043–4050. 10.1023/A:1004672117361. - DOI
-
- Grass R.; Athanassiou E.; Stark W.. Methods and Devices for Flame Spray Pyrolysis. U.S. Patent US8,182,573, 2012.
Grants and funding
LinkOut - more resources
Full Text Sources

