Anticancer Effects of Plasma-Treated Water Solutions from Clinically Approved Infusion Liquids Supplemented with Organic Molecules
- PMID: 37744835
- PMCID: PMC10515361
- DOI: 10.1021/acsomega.3c04061
Anticancer Effects of Plasma-Treated Water Solutions from Clinically Approved Infusion Liquids Supplemented with Organic Molecules
Abstract
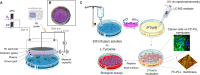
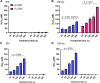
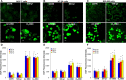
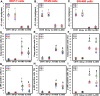
Water solutions treated by cold atmospheric plasmas (CAPs) currently stand out in the field of cancer treatment as sources of exogenous blends of reactive oxygen and nitrogen species (RONS). It is well known that the balance of RONS inside both eukaryotic and prokaryotic cells is directly involved in physiological as well as pathological pathways. Also, organic molecules including phenols could exert promising anticancer effects, mostly attributed to their pro-oxidant ability in vitro and in vivo to generate RONS like O2-, H2O2, and a mixture of potentially cytotoxic compounds. By our vision of combining the efficacy of plasma-produced RONS and the use of organic molecules, we could synergistically attack cancer cells; yet, so far, this combination, to the best of our knowledge, has been completely unexplored. In this study, l-tyrosine, an amino acid with a phenolic side chain, is added to a physiological solution, often used in clinical practice (SIII) to be exposed to plasma. The efficacy of the gas plasma-oxidized SIII solution, containing tyrosine, was evaluated on four cancer cell lines selected from among tumors with poor prognosis (SHSY-5Y, MCF-7, HT-29, and SW-480). The aim was to induce tumor toxicity and trigger apoptosis pathways. The results clearly indicate that the plasma-treated water solution (PTWS) reduced cell viability and oxygen uptake due to an increase in intracellular ROS levels and activation of apoptosis pathways in all investigated cancer cells, which may be related to the activation of the mitochondrial-mediated and p-JNK/caspase-3 signaling pathways. This research offers improved knowledge about the physiological mechanisms underlying cancer treatment and a valid method to set up a prompt, adequate, and effective cancer treatment in the clinic.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Enhanced Generation of Reactive Species by Cold Plasma in Gelatin Solutions for Selective Cancer Cell Death.ACS Appl Mater Interfaces. 2020 Oct 21;12(42):47256-47269. doi: 10.1021/acsami.0c12930. Epub 2020 Oct 6. ACS Appl Mater Interfaces. 2020. PMID: 33021783
-
Direct Sensing of Superoxide and Its Relatives Reactive Oxygen and Nitrogen Species in Phosphate Buffers during Cold Atmospheric Plasmas Exposures.Anal Chem. 2022 Apr 12;94(14):5555-5565. doi: 10.1021/acs.analchem.1c04998. Epub 2022 Mar 28. Anal Chem. 2022. PMID: 35343678
-
Cancer-specific cytotoxicity of Ringer's acetate solution irradiated by cold atmospheric pressure plasma.Free Radic Res. 2023 Feb;57(2):91-104. doi: 10.1080/10715762.2023.2201390. Epub 2023 Apr 17. Free Radic Res. 2023. PMID: 37067923
-
Reactive Oxygen and Nitrogen Species (RONS) and Cytokines-Myokines Involved in Glucose Uptake and Insulin Resistance in Skeletal Muscle.Cells. 2022 Dec 11;11(24):4008. doi: 10.3390/cells11244008. Cells. 2022. PMID: 36552772 Free PMC article. Review.
-
Reactive Oxygen and Nitrogen Species-Induced Protein Modifications: Implication in Carcinogenesis and Anticancer Therapy.Cancer Res. 2018 Nov 1;78(21):6040-6047. doi: 10.1158/0008-5472.CAN-18-0980. Epub 2018 Oct 16. Cancer Res. 2018. PMID: 30327380 Review.
Cited by
-
Comparing Redox and Intracellular Signalling Responses to Cold Plasma in Wound Healing and Cancer.Curr Issues Mol Biol. 2024 May 17;46(5):4885-4923. doi: 10.3390/cimb46050294. Curr Issues Mol Biol. 2024. PMID: 38785562 Free PMC article. Review.
-
Chemical Analysis of Plasma-Activated Culture Media by Ion Chromatography.Pharmaceuticals (Basel). 2025 Feb 1;18(2):199. doi: 10.3390/ph18020199. Pharmaceuticals (Basel). 2025. PMID: 40006013 Free PMC article.
-
Human head and neck cancer cell lines response to cold atmospheric plasma activated media is affected by the chemistry of culture media.Heliyon. 2024 Dec 25;11(1):e41458. doi: 10.1016/j.heliyon.2024.e41458. eCollection 2025 Jan 15. Heliyon. 2024. PMID: 39866438 Free PMC article.
References
-
- Fridman G.; Friedman G.; Gutsol A.; Shekhter A. B.; Vasilets V. N.; Fridman A. Applied plasma medicine. Plasma Processes Polym. 2008, 5, 503–533. 10.1002/ppap.200700154. - DOI
-
- Laroussi M. From Killing Bacteria to Destroying Cancer Cells: 20 years of Plasma Medicine. Plasma Processes Polym. 2014, 11, 1138–1141. 10.1002/ppap.201400152. - DOI
-
- Schlegel J.; Köritzer J.; Boxhammer V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. 10.1016/j.cpme.2013.08.001. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

