Phenotypic assay for cytotoxicity assessment of Balamuthia mandrillaris against human neurospheroids
- PMID: 37744897
- PMCID: PMC10513763
- DOI: 10.3389/fmicb.2023.1190530
Phenotypic assay for cytotoxicity assessment of Balamuthia mandrillaris against human neurospheroids
Abstract
Introduction: The phenotypic screening of drugs against Balamuthia mandrillaris, a neuropathogenic amoeba, involves two simultaneous phases: an initial step to test amoebicidal activity followed by an assay for cytotoxicity to host cells. The emergence of three-dimensional (3D) cell cultures has provided a more physiologically relevant model than traditional 2D cell culture for studying the pathogenicity of B. mandrillaris. However, the measurement of ATP, a critical indicator of cell viability, is complicated by the overgrowth of B. mandrillaris in coculture with host cells during drug screening, making it challenging to differentiate between amoebicidal activity and drug toxicity to human cells.
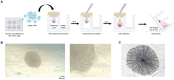
Methods: To address this limitation, we introduce a novel assay that utilizes three-dimensional hanging spheroid plates (3DHSPs) to evaluate both activities simultaneously on a single platform.
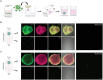
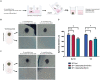
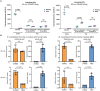
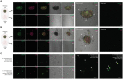
Results and discussion: Our study showed that the incubation of neurospheroids with clinically isolated B. mandrillaris trophozoites resulted in a loss of neurospheroid integrity, while the ATP levels in the neurospheroids decreased over time, indicating decreased host cell viability. Conversely, ATP levels in isolated trophozoites increased, indicating active parasite metabolism. Our findings suggest that the 3DHSP-based assay can serve as an endpoint for the phenotypic screening of drugs against B. mandrillaris, providing a more efficient and accurate approach for evaluating both parasite cytotoxicity and viability.
Keywords: Balamuthia mandrillaris; cytotoxicity; drug discovery; granulomatous amoebic encephalitis; neglected disease; neurospheroid; tropical disease.
Copyright © 2023 Whangviboonkij, Pengsart, Chen, Han, Park and Kulkeaw.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Biedler J. L., Roffler-Tarlov S., Schachner M., Freedman L. S. (1978). Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 38, 3751–3757. PMID: - PubMed
LinkOut - more resources
Full Text Sources

