AI-driven pan-proteome analyses reveal insights into the biohydrometallurgical properties of Acidithiobacillia
- PMID: 37744906
- PMCID: PMC10512742
- DOI: 10.3389/fmicb.2023.1243987
AI-driven pan-proteome analyses reveal insights into the biohydrometallurgical properties of Acidithiobacillia
Abstract
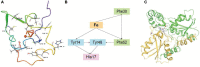
Microorganism-mediated biohydrometallurgy, a sustainable approach for metal recovery from ores, relies on the metabolic activity of acidophilic bacteria. Acidithiobacillia with sulfur/iron-oxidizing capacities are extensively studied and applied in biohydrometallurgy-related processes. However, only 14 distinct proteins from Acidithiobacillia have experimentally determined structures currently available. This significantly hampers in-depth investigations of Acidithiobacillia's structure-based biological mechanisms pertaining to its relevant biohydrometallurgical processes. To address this issue, we employed a state-of-the-art artificial intelligence (AI)-driven approach, with a median model confidence of 0.80, to perform high-quality full-chain structure predictions on the pan-proteome (10,458 proteins) of the type strain Acidithiobacillia. Additionally, we conducted various case studies on de novo protein structural prediction, including sulfate transporter and iron oxidase, to demonstrate how accurate structure predictions and gene co-occurrence networks can contribute to the development of mechanistic insights and hypotheses regarding sulfur and iron utilization proteins. Furthermore, for the unannotated proteins that constitute 35.8% of the Acidithiobacillia proteome, we employed the deep-learning algorithm DeepFRI to make structure-based functional predictions. As a result, we successfully obtained gene ontology (GO) terms for 93.6% of these previously unknown proteins. This study has a significant impact on improving protein structure and function predictions, as well as developing state-of-the-art techniques for high-throughput analysis of large proteomic data.
Keywords: Acidithiobacillia; biohydrometallurgy; gene co-occurrence network analysis; protein structure prediction; proteome.
Copyright © 2023 Li, Zhou, Jiang, Liu, Meng, Luo, He and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Comparative genomics sheds light on transcription factor-mediated regulation in the extreme acidophilic Acidithiobacillia representatives.Res Microbiol. 2024 Jan-Feb;175(1-2):104135. doi: 10.1016/j.resmic.2023.104135. Epub 2023 Sep 9. Res Microbiol. 2024. PMID: 37678513
-
Integrative Genomics Sheds Light on Evolutionary Forces Shaping the Acidithiobacillia Class Acidophilic Lifestyle.Front Microbiol. 2022 Feb 15;12:822229. doi: 10.3389/fmicb.2021.822229. eCollection 2021. Front Microbiol. 2022. PMID: 35242113 Free PMC article.
-
Electrochemical Applications in Metal Bioleaching.Adv Biochem Eng Biotechnol. 2019;167:327-359. doi: 10.1007/10_2017_36. Adv Biochem Eng Biotechnol. 2019. PMID: 29224081
-
Artificial Intelligence in De novo Drug Design: Are We Still There?Curr Top Med Chem. 2022;22(30):2483-2492. doi: 10.2174/1568026623666221017143244. Curr Top Med Chem. 2022. PMID: 36263480 Review.
-
Obtaining genetics insights from deep learning via explainable artificial intelligence.Nat Rev Genet. 2023 Feb;24(2):125-137. doi: 10.1038/s41576-022-00532-2. Epub 2022 Oct 3. Nat Rev Genet. 2023. PMID: 36192604 Review.
Cited by
-
Revolutionary Point-of-Care Wearable Diagnostics for Early Disease Detection and Biomarker Discovery through Intelligent Technologies.Adv Sci (Weinh). 2024 Sep;11(36):e2400595. doi: 10.1002/advs.202400595. Epub 2024 Jul 3. Adv Sci (Weinh). 2024. PMID: 38958517 Free PMC article. Review.
References
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

