Development of a specific MPXV antigen detection immunodiagnostic assay
- PMID: 37744911
- PMCID: PMC10516133
- DOI: 10.3389/fmicb.2023.1243523
Development of a specific MPXV antigen detection immunodiagnostic assay
Abstract
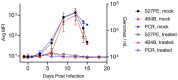
Human monkeypox (mpox) has recently become a global public health emergency; however, assays that detect mpox infection are not widely available, largely due to cross-reactivity within the Orthopoxvirus genus. Immunoassay development was largely confined to researchers who focus on biothreats and endemic areas (Central and West Africa) until the 2022 outbreak. As was noted in the COVID-19 pandemic, antigen detection assays, integrated with molecular assays, are necessary to help curb the spread of disease. Antigen-detecting immunoassays offer the advantage of providing results ranging from within min to h and in lateral flow formats; they can be deployed for point-of-care, home, or field use. This study reports the development of an mpox-specific antigen detection immunoassay developed on a multiplexed, magnetic-bead-based platform utilizing reagents from all research sectors (commercial, academic, and governmental). Two semi-quantitative assays were developed in parallel and standardized with infectious mpox virus (MPXV) cell culture fluid and MPXV-positive non-human primate (NHP) sera samples. These assays could detect viral antigens in serum, were highly specific toward MPXV as compared to other infectious orthopoxviruses (vaccinia virus, cowpox virus, and camelpox virus), and exhibited a correlation with quantitative PCR results from an NHP study. Access to a toolbox of assays for mpox detection will be key for identifying cases and ensuring proper treatment, as MPXV is currently a global traveler.
Keywords: Magpix; antigen assay; clade-specific; immunofluorescence; mpox.
Copyright © 2023 Davis, Payne, Olguin, Sanders, Clements, Stefan, Williams, Hooper, Huggins, Mucker and Ricks.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Evaluation of a multiplexed immunoassay for assessing long-term humoral immunity Orthopoxviruses.Vaccine. 2024 Dec 2;42(26):126453. doi: 10.1016/j.vaccine.2024.126453. Epub 2024 Oct 18. Vaccine. 2024. PMID: 39426286
-
Synthetic modified vaccinia Ankara vaccines confer cross-reactive and protective immunity against mpox virus.Commun Med (Lond). 2024 Feb 16;4(1):19. doi: 10.1038/s43856-024-00443-9. Commun Med (Lond). 2024. PMID: 38366141 Free PMC article.
-
Defining antigen targets to dissect vaccinia virus and monkeypox virus-specific T cell responses in humans.Cell Host Microbe. 2022 Dec 14;30(12):1662-1670.e4. doi: 10.1016/j.chom.2022.11.003. Epub 2022 Dec 3. Cell Host Microbe. 2022. PMID: 36463861 Free PMC article.
-
Human monkeypox virus in the shadow of the COVID-19 pandemic.J Infect Public Health. 2023 Aug;16(8):1149-1157. doi: 10.1016/j.jiph.2023.05.013. Epub 2023 May 13. J Infect Public Health. 2023. PMID: 37269693 Free PMC article. Review.
-
Mpox Virus: Its Molecular Evolution and Potential Impact on Viral Epidemiology.Viruses. 2023 Apr 18;15(4):995. doi: 10.3390/v15040995. Viruses. 2023. PMID: 37112975 Free PMC article. Review.
Cited by
-
Mpox disease, diagnosis, and point of care platforms.Bioeng Transl Med. 2025 Jan 2;10(3):e10733. doi: 10.1002/btm2.10733. eCollection 2025 May. Bioeng Transl Med. 2025. PMID: 40385539 Free PMC article. Review.
-
Monkeypox virus A29L protein as the target for specific diagnosis and serological analysis.Appl Microbiol Biotechnol. 2024 Nov 21;108(1):522. doi: 10.1007/s00253-024-13361-6. Appl Microbiol Biotechnol. 2024. PMID: 39570405 Free PMC article.
-
Early detection of human Mpox: A comparative study by using machine learning and deep learning models with ensemble approach.Digit Health. 2025 Jun 4;11:20552076251344135. doi: 10.1177/20552076251344135. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40496715 Free PMC article.
-
A Label-free Optical Biosensor-Based Point-of-Care Test for the Rapid Detection of Monkeypox Virus.medRxiv [Preprint]. 2024 Jul 5:2024.07.03.24309903. doi: 10.1101/2024.07.03.24309903. medRxiv. 2024. Update in: Biosens Bioelectron. 2025 Feb 1;269:116932. doi: 10.1016/j.bios.2024.116932. PMID: 39006424 Free PMC article. Updated. Preprint.
-
Preparation and application evaluation of monoclonal antibodies against Monkeypox virus A29 protein.Front Microbiol. 2025 Jan 31;16:1547021. doi: 10.3389/fmicb.2025.1547021. eCollection 2025. Front Microbiol. 2025. PMID: 39959162 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

