Micromonospora profundi TRM 95458 converts glycerol to a new osmotic compound
- PMID: 37744923
- PMCID: PMC10513789
- DOI: 10.3389/fmicb.2023.1236906
Micromonospora profundi TRM 95458 converts glycerol to a new osmotic compound
Abstract
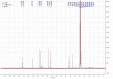
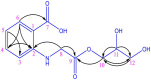
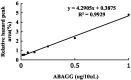
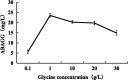
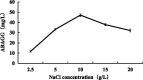
Plant growth and agricultural productivity was greatly limited by soil salinity and alkalization. The application of salt-tolerant rhizobacteria could effectively improve plant tolerance to saline-alkali stress. Micromonospora profundi TRM 95458 was obtained from the rhizosphere of chickpea (Cicer arietinum L.) as a moderate salt-tolerant rhizobacteria. A new osmotic compound (ABAGG) was isolated from the fermentation broth of M. profundi TRM 95458. The chemical structure of the new compound was elucidated by analyzing nuclear magnetic resonance (NMR) and high-resolution mass (HRMS) data. M. profundi TRM 95458 could convert glycerol into ABAGG. The accumulation of ABAGG varied depending on the amount of glycerol and glycine added to the fermentation medium. In addition, the concentration of NaCl affected the ABAGG content obviously. The highest yield of ABAGG was observed when the salt content of the fermentation medium was 10 g/L. The study indicated that salt stress led to the accumulation of ABAGG using glycerol and glycine as substrates, suggesting ABAGG might aid in the survival and adaptation of the strain in saline-alkaline environments as a new osmotic compound.
Keywords: ABAGG; Micromonospora profundi TRM 95458; compatible solutes; conversion of glycerol; salt-tolerant rhizobacteria.
Copyright © 2023 Lu, Shen, Wang and Wan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Amro A., Harb S., Farghaly K. A., Ali M. M. F., Mohammed A. G., Mourad A. M. I., et al. . (2022). Growth responses and genetic variation among highly ecologically diverse spring wheat genotypes grown under seawater stress. Front. Plant Sci. 13:996538. doi: 10.3389/fpls.2022.996538, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

