Resistome-based surveillance identifies ESKAPE pathogens as the predominant gram-negative organisms circulating in veterinary hospitals
- PMID: 37744932
- PMCID: PMC10513425
- DOI: 10.3389/fmicb.2023.1252216
Resistome-based surveillance identifies ESKAPE pathogens as the predominant gram-negative organisms circulating in veterinary hospitals
Abstract
Introduction: Healthcare-associated infections (HCAIs) associated with extended-spectrum cephalosporin-resistant gram-negative (ESC-R GN) bacteria are an emerging concern in veterinary hospitals, especially in companion animal intensive care units (ICUs).
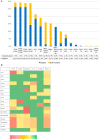
Methods: To understand the molecular epidemiology of ESC-R GN isolates in two veterinary hospitals (equine and small animal), a 6-month pilot study was performed during which fecal and environmental samples were obtained twice from selected patients, upon ICU admission and after 48 h of hospitalization. In total, 295 ESC-R GNs were analyzed using the Acuitas Resistome® Test (OpGen, Maryland, US), a PCR-based assay screening for 50 antimicrobial resistance gene families encoding for production of extended-spectrum beta-lactamase (ESBLs), TEM/SHV/OXA or AmpC beta-lactamases and carbapenemases. Combining organism identification and antimicrobial susceptibility data to genotyping results, unique "Acuitas profiles" were generated that can be used for fast typing the isolates and tracking transmission events.
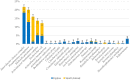
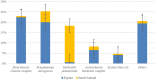
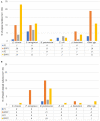
Results: ESKAPE GN pathogens were the most prevalent ESC-R GN isolates circulating in both the small animal and equine hospitals, consisting of Enterobacter cloacae complex (21.7%), Pseudomonas aeruginosa (20%), Klebsiella pneumoniae (15.9%), and Acinetobacter baumannii complex (13.6%) followed by Escherichia coli (12.2%), most harboring a combination of genes encoding for beta-lactamases and ESBLs. Some ESKAPE genotypes showed likely intra-hospital transmission, including E. cloacae (two genotypes, one carrying SHV4, SHV5, and TEM7 and the other TEM1, TEM3, and TEM7 enzymes) in the equine and K. pneumoniae (SHV1, SHV5, and DHA1-positive) in the small animal ICUs, respectively. Furthermore, P. aeruginosa (carrying OXA-50), A. baumannii complex (OXA-51), and E. coli (CTX-M-1) genotypes were isolated across both hospitals, suggesting possible transfer mediated via movement of staff and students. Importantly, isolates carrying transmissible resistance to last-resort antimicrobials (i.e. carbapenems) were identified within the hospital environments, consisting of three environmental Acinetobacter spp. harboring blaOXA - 23 and one clinical E. coli with blaOXA - 48.
Conclusion: We describe the widespread occurrence of ESKAPE gram-negative organisms in veterinary ICU patients and hospital environments. Findings from this project provide baseline data on the epidemiology of ESKAPE pathogens in veterinary settings, which can inform infection control policies to aid in patient management and prevent transmission of nosocomial infections associated with these pathogens.
Keywords: ESKAPE; companion animals; gram-negative; infection control; intensive care unit (ICU); surveillance; veterinary; veterinary hospitals.
Copyright © 2023 Zendri, Isgren, Devaney, Schmidt, Rankin and Timofte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Gram-negative ESKAPE bacteria bloodstream infections in patients during the COVID-19 pandemic.PeerJ. 2023 Mar 29;11:e15007. doi: 10.7717/peerj.15007. eCollection 2023. PeerJ. 2023. PMID: 37013147 Free PMC article.
-
Gram-negative bacteria as causative agents of ventilator-associated pneumonia and their respective resistance mechanisms.J Chemother. 2020 Nov;32(7):344-358. doi: 10.1080/1120009X.2020.1793594. Epub 2020 Jul 30. J Chemother. 2020. PMID: 32729399
-
Extended spectrum beta-lactamase mediated resistance in carriage and clinical gram-negative ESKAPE bacteria: a comparative study between a district and tertiary hospital in South Africa.Antimicrob Resist Infect Control. 2018 Nov 14;7:134. doi: 10.1186/s13756-018-0423-0. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 30473784 Free PMC article.
-
Retrospective Analysis on Antimicrobial Resistance Trends and Prevalence of β-lactamases in Escherichia coli and ESKAPE Pathogens Isolated from Arabian Patients during 2000-2020.Microorganisms. 2020 Oct 21;8(10):1626. doi: 10.3390/microorganisms8101626. Microorganisms. 2020. PMID: 33096921 Free PMC article. Review.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
Impact of Antimicrobial Resistance of Pseudomonas aeruginosa in Urine of Small Companion Animals in Global Context: Comprehensive Analysis.Vet Sci. 2025 Feb 11;12(2):157. doi: 10.3390/vetsci12020157. Vet Sci. 2025. PMID: 40005917 Free PMC article. Review.
-
PRO: Environmental microbiological surveillance does support infection control in veterinary hospitals.JAC Antimicrob Resist. 2024 Aug 1;6(4):dlae113. doi: 10.1093/jacamr/dlae113. eCollection 2024 Aug. JAC Antimicrob Resist. 2024. PMID: 39091688 Free PMC article. No abstract available.
-
Antimicrobial resistance of pet-derived bacteria in China, 2000-2020.Antimicrob Agents Chemother. 2025 Mar 26;69(5):e0165724. doi: 10.1128/aac.01657-24. Online ahead of print. Antimicrob Agents Chemother. 2025. PMID: 40135877 Free PMC article. Review.
-
Genomic Characterization of Carbapenem-Resistant Acinetobacter baumannii (OXA-23) and Klebsiella pneumoniae (KPC-2) Causing Hospital-Acquired Infections in Dogs.Antibiotics (Basel). 2025 Jun 6;14(6):584. doi: 10.3390/antibiotics14060584. Antibiotics (Basel). 2025. PMID: 40558175 Free PMC article.
-
Rapid typing of Klebsiella pneumoniae and Pseudomonas aeruginosa by Fourier-transform Infrared spectroscopy informs infection control in veterinary settings.Front Microbiol. 2024 Feb 2;15:1334268. doi: 10.3389/fmicb.2024.1334268. eCollection 2024. Front Microbiol. 2024. PMID: 38371930 Free PMC article.
References
-
- Bernal-Rosas Y., Osorio-Muñoz K., Torres-García O. (2015). Pseudomonas aeruginosa: an emerging nosocomial trouble in veterinary. Rev. MVZ Córdoba. 20, 4937–4946. 10.21897/rmvz.9 - DOI
-
- Cassini A., Högberg L. D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G. S., et al. . (2019). Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66. 10.1016/S1473-3099(18)30605-4 - DOI - PMC - PubMed
-
- CLSI (2018a). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. 4th edition, Wayne, PA: VET08.
LinkOut - more resources
Full Text Sources
Miscellaneous

