The impact of adjuvant EGFR-TKIs and 14-gene molecular assay on stage I non-small cell lung cancer with sensitive EGFR mutations
- PMID: 37745018
- PMCID: PMC10511786
- DOI: 10.1016/j.eclinm.2023.102205
The impact of adjuvant EGFR-TKIs and 14-gene molecular assay on stage I non-small cell lung cancer with sensitive EGFR mutations
Abstract
Background: Currently, the role of EGFR-TKIs as adjuvant therapy for stage I, especially IA NSCLC, after surgical resection remains unclear. We aimed to compare the effect of adjuvant EGFR-TKIs with observation in such patients by incorporating an established 14-gene molecular assay for risk stratification.
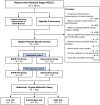
Methods: This retrospective cohort study was conducted at the First Affiliated Hospital of Guangzhou Medical University (Study ID: ChNCRCRD-2022-GZ01). From March 2013 to February 2019, completely resected stage I NSCLC (8th TNM staging) patients with sensitive EGFR mutation were included. Patients with eligible samples for molecular risk stratification were subjected to the 14-gene prognostic assay. Inverse probability of treatment weighting (IPTW) was employed to minimize imbalances in baseline characteristics.
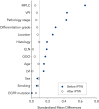
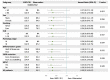
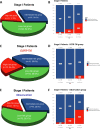
Findings: A total of 227 stage I NSCLC patients were enrolled, with 55 in EGFR-TKI group and 172 in the observation group. The median duration of follow-up was 78.4 months. After IPTW, the 5-year DFS (HR = 0.30, 95% CI, 0.14-0.67; P = 0.003) and OS (HR = 0.26, 95% CI, 0.07-0.96; P = 0.044) of the EGFR-TKI group were significantly better than the observation group. For subgroup analyses, adjuvant EGFR-TKIs were associated with favorable 5-year DFS rates in both IA (100.0% vs. 84.5%; P = 0.007), and IB group (98.8% vs. 75.3%; P = 0.008). The 14-gene assay was performed in 180 patients. Among intermediate-high-risk patients, EGFR-TKIs were associated with a significant improvement in 5-year DFS rates compared to observation (96.0% vs. 70.5%; P = 0.012), while no difference was found in low-risk patients (100.0% vs. 94.9%; P = 0.360).
Interpretation: Our study suggested that adjuvant EGFR-TKI might improve DFS and OS of stage IA and IB EGFR-mutated NSCLC, and the 14-gene molecular assay could help patients that would benefit the most from treatment.
Funding: This work was supported by China National Science Foundation (82022048, 82373121).
Keywords: Adjuvant therapy; EGFR-TKI; Non–small-cell lung cancer; Risk stratification; Stage I.
© 2023 The Author(s).
Conflict of interest statement
There are no conflicts of interest to declare.
Figures






References
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Chansky K., Detterbeck F.C., Nicholson A.G., et al. The IASLC lung cancer staging project: external validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2017;12(7):1109–1121. - PubMed
-
- Goldstraw P., Chansky K., Crowley J., et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. - PubMed
-
- Park J.H., Lee C.-T., Lee H.W., Baek H.J., Zo J.I., Shim Y.M. Postoperative adjuvant chemotherapy for stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2005;27(6):1086–1091. - PubMed
-
- Pignon J.-P., Tribodet H., Scagliotti G.V., et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

