Characterization of long COVID temporal sub-phenotypes by distributed representation learning from electronic health record data: a cohort study
- PMID: 37745021
- PMCID: PMC10511779
- DOI: 10.1016/j.eclinm.2023.102210
Characterization of long COVID temporal sub-phenotypes by distributed representation learning from electronic health record data: a cohort study
Abstract
Background: Characterizing Post-Acute Sequelae of COVID (SARS-CoV-2 Infection), or PASC has been challenging due to the multitude of sub-phenotypes, temporal attributes, and definitions. Scalable characterization of PASC sub-phenotypes can enhance screening capacities, disease management, and treatment planning.
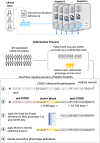
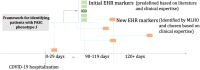
Methods: We conducted a retrospective multi-centre observational cohort study, leveraging longitudinal electronic health record (EHR) data of 30,422 patients from three healthcare systems in the Consortium for the Clinical Characterization of COVID-19 by EHR (4CE). From the total cohort, we applied a deductive approach on 12,424 individuals with follow-up data and developed a distributed representation learning process for providing augmented definitions for PASC sub-phenotypes.
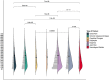
Findings: Our framework characterized seven PASC sub-phenotypes. We estimated that on average 15.7% of the hospitalized COVID-19 patients were likely to suffer from at least one PASC symptom and almost 5.98%, on average, had multiple symptoms. Joint pain and dyspnea had the highest prevalence, with an average prevalence of 5.45% and 4.53%, respectively.
Interpretation: We provided a scalable framework to every participating healthcare system for estimating PASC sub-phenotypes prevalence and temporal attributes, thus developing a unified model that characterizes augmented sub-phenotypes across the different systems.
Funding: Authors are supported by National Institute of Allergy and Infectious Diseases, National Institute on Aging, National Center for Advancing Translational Sciences, National Medical Research Council, National Institute of Neurological Disorders and Stroke, European Union, National Institutes of Health, National Center for Advancing Translational Sciences.
Keywords: COVID-19; Electronic health records; PASC; Post-acute sequelae of SARS-CoV-2; SARS-CoV-2.
© 2023 The Author(s).
Conflict of interest statement
Riccardo Bellazzi is shareholder of Biomeris s. r.l. Gilbert Omenn holds patents for U.S. Application No. 16/169,048 Filed: 24-October- 2018 and License 2023–0632 with Radial Therapeutics, Inc.; Invention Disclosure No. 2022-382.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

