Risk-stratified monitoring for thiopurine toxicity in immune-mediated inflammatory diseases: prognostic model development, validation, and, health economic evaluation
- PMID: 37745026
- PMCID: PMC10514402
- DOI: 10.1016/j.eclinm.2023.102213
Risk-stratified monitoring for thiopurine toxicity in immune-mediated inflammatory diseases: prognostic model development, validation, and, health economic evaluation
Abstract
Background: Patients established on thiopurines (e.g., azathioprine) are recommended to undergo three-monthly blood tests for the early detection of blood, liver, or kidney toxicity. These side-effects are uncommon during long-term treatment. We developed a prognostic model that could be used to inform risk-stratified decisions on frequency of monitoring blood-tests during long-term thiopurine treatment, and, performed health-economic evaluation of alternate monitoring intervals.
Methods: This was a retrospective cohort study set in the UK primary-care. Data from the Clinical Practice Research Datalink Aurum and Gold formed development and validation cohorts, respectively. People age ≥18 years, diagnosed with an immune mediated inflammatory disease, prescribed thiopurine by their general practitioner for at-least six-months between January 1, 2007 and December 31, 2019 were eligible. The outcome was thiopurine discontinuation with abnormal blood-test results. Patients were followed up from six-months after first primary-care thiopurine prescription to up to five-years. Penalised Cox regression developed the risk equation. Multiple imputation handled missing predictor data. Calibration and discrimination assessed model performance. A mathematical model evaluated costs and quality-adjusted life years associated with lengthening the interval between blood-tests.
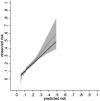
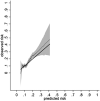
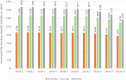
Findings: Data from 5982 (405 events over 16,117 person-years) and 3573 (269 events over 9075 person-years) participants were included in the development and validation cohorts, respectively. Fourteen candidate predictors (21 parameters) were included. The optimism adjusted R2 and Royston D statistic in development data were 0.11 and 0.76, respectively. The calibration slope and Royston D statistic (95% Confidence Interval) in the validation data were 1.10 (0.84-1.36) and 0.72 (0.52-0.92), respectively. A 2-year period between monitoring blood-test was most cost-effective in all deciles of predicted risk but the gain between monitoring annually or biennially reduced in higher risk deciles.
Interpretation: This prognostic model requires information that is readily available during routine clinical care and may be used to risk-stratify blood-test monitoring for thiopurine toxicity. These findings should be considered by specialist societies when recommending blood monitoring during thiopurine prescription to bring about sustainable and equitable change in clinical practice.
Funding: National Institute for Health and Care Research.
Keywords: Drug toxicity prediction; Inflammatory bowel disease; Thiopurine.
© 2023 The Author(s).
Conflict of interest statement
A.A. has received Institutional research grants from AstraZeneca and Oxford Immunotech; and personal fees from UpToDate (royalty), Springer (royalty), Cadilla Pharmaceuticals (lecture fees), NGM Bio (consulting), Limbic (consulting) and personal fees from Inflazome (consulting) unrelated to the work. GPA has received consulting fees from Abbott Products, Albireo Pharma, Amryth, AstraZeneca, BenevolentAI Bio, Clinipace, DNDI, GlaxoSmithKline, Merck, NuCANA, Puretech, Pfizer, Roche Diagnostics, Servier Pharmaceuticals, W.L. Gore & Associates paid to the University of Nottingham unrelated to the work. CPF has received Consultancy/Advisory board fees from Abbvie, GenMab, Incyte, Morphosys, Roche, Takeda, Ono, Kite/Gilead, BMS/Celgene, BTG/Veriton and departmental research funding from BeiGene unrelated to the work. The other authors have no conflict of interest to declare. Keele University has received research funding for CDM from NIHR, MRC, Versus Arthritis and BMS. CDM is Director of the NIHR School for Primary Care Research. HCW worked for the National Institute of Health Research 2015–2021. He had no part to play in the funding of this study.
Figures


References
-
- Raine T., Bonovas S., Burisch J., et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. - PubMed
-
- Fanouriakis A., Tziolos N., Bertsias G., Boumpas D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann Rheum Dis. 2021;80(1):14–25. - PubMed
LinkOut - more resources
Full Text Sources

