Drug repurposing for Alzheimer's disease from 2012-2022-a 10-year literature review
- PMID: 37745051
- PMCID: PMC10512468
- DOI: 10.3389/fphar.2023.1257700
Drug repurposing for Alzheimer's disease from 2012-2022-a 10-year literature review
Abstract
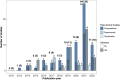
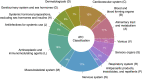
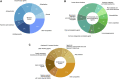
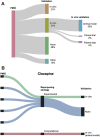
Background: Alzheimer's disease (AD) is a debilitating neurodegenerative condition with few treatment options available. Drug repurposing studies have sought to identify existing drugs that could be repositioned to treat AD; however, the effectiveness of drug repurposing for AD remains unclear. This review systematically analyzes the progress made in drug repurposing for AD throughout the last decade, summarizing the suggested drug candidates and analyzing changes in the repurposing strategies used over time. We also examine the different types of data that have been leveraged to validate suggested drug repurposing candidates for AD, which to our knowledge has not been previous investigated, although this information may be especially useful in appraising the potential of suggested drug repurposing candidates. We ultimately hope to gain insight into the suggested drugs representing the most promising repurposing candidates for AD. Methods: We queried the PubMed database for AD drug repurposing studies published between 2012 and 2022. 124 articles were reviewed. We used RxNorm to standardize drug names across the reviewed studies, map drugs to their constituent ingredients, and identify prescribable drugs. We used the Anatomical Therapeutic Chemical (ATC) Classification System to group drugs. Results: 573 unique drugs were proposed for repurposing in AD over the last 10 years. These suggested repurposing candidates included drugs acting on the nervous system (17%), antineoplastic and immunomodulating agents (16%), and drugs acting on the cardiovascular system (12%). Clozapine, a second-generation antipsychotic medication, was the most frequently suggested repurposing candidate (N = 6). 61% (76/124) of the reviewed studies performed a validation, yet only 4% (5/124) used real-world data for validation. Conclusion: A large number of potential drug repurposing candidates for AD has accumulated over the last decade. However, among these drugs, no single drug has emerged as the top candidate, making it difficult to establish research priorities. Validation of drug repurposing hypotheses is inconsistently performed, and real-world data has been critically underutilized for validation. Given the urgent need for new AD therapies, the utility of real-world data in accelerating identification of high-priority candidates for AD repurposing warrants further investigation.
Keywords: Alzheimer’s disease; drug repurposing; electronic health records; real-world data; validation.
Copyright © 2023 Grabowska, Huang, Wen, Li and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Anatomical Therapeutic Chemical (ATC) (2022). Anatomical therapeutic chemical (ATC) classification. Available at: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed October 5, 2022).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

