In vivo tracking transplanted cardiomyocytes derived from human induced pluripotent stem cells using nuclear medicine imaging
- PMID: 37745108
- PMCID: PMC10512708
- DOI: 10.3389/fcvm.2023.1261330
In vivo tracking transplanted cardiomyocytes derived from human induced pluripotent stem cells using nuclear medicine imaging
Abstract
Introduction: Transplantation of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) is a promising treatment for heart failure. Information on long-term cell engraftment after transplantation is clinically important. However, clinically applicable evaluation methods have not yet been established.
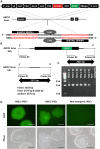
Methods: In this study, to noninvasively assess transplanted cell engraftment, human SLC5A5, which encodes a sodium/iodide symporter (NIS) that transports radioactive tracers such as 125I, 18F-tetrafluoroborate (TFB), and 99mTc-pertechnetate (99mTcO4-), was transduced into human induced pluripotent stem cells (iPSCs), and nuclear medicine imaging was used to track engrafted human iPSC-CMs.
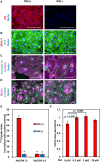
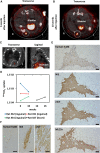
Results: To evaluate the pluripotency of NIS-expressing human iPSCs, they were subcutaneously transplanted into immunodeficient rats. Teratomas were detected by 99mTcO4- single photon emission computed tomography (SPECT/CT) imaging. NIS expression and the uptake ability of 125I were maintained in purified human iPSC-CMs. NIS-expressing human iPSC-CMs transplanted into immunodeficient rats could be detected over time using 99mTcO4- SPECT/CT imaging. Unexpectedly, NIS expression affected cell proliferation of human iPSCs and iPSC-derived cells.
Discussion: Such functionally designed iPSC-CMs have potential clinical applications as a noninvasive method of grafted cell evaluation, but further studies are needed to determine the effects of NIS transduction on cellular characteristics and functions.
Keywords: cell-based therapy; human induced pluripotent stem cell-derived cardiomyocytes; in vivo imaging; single photon emission computed tomography; sodium/iodide symporter.
© 2023 Saito, Nose, Iida, Akazawa, Kanno, Fujimoto, Sasaki, Akehi, Higuchi, Akagi, Yoshida, Miyoshi, Ito and Nakamura.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–e1032. 10.1161/cir.0000000000001063 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

