A combination of strongly associated prothrombotic single nucleotide polymorphisms could efficiently predict venous thrombosis risk
- PMID: 37745125
- PMCID: PMC10511882
- DOI: 10.3389/fcvm.2023.1224462
A combination of strongly associated prothrombotic single nucleotide polymorphisms could efficiently predict venous thrombosis risk
Abstract
Background: Venous thrombosis (VT) is multifactorial trait that contributes to the global burden of cardiovascular diseases. Although abundant single nucleotide polymorphisms (SNPs) provoke the susceptibility of an individual to VT, research has found that the five most strongly associated SNPs, namely, rs6025 (F5 Leiden), rs2066865 (FGG), rs2036914 (F11), rs8176719 (ABO), and rs1799963 (F2), play the greatest role. Association and risk prediction models are rarely established by using merely the five strongly associated SNPs. This study aims to explore the combined VT risk predictability of the five SNPs and well-known non-genetic VT risk factors such as aging and obesity in the Hungarian population.
Methods: SNPs were genotyped in the VT group (n = 298) and control group (n = 400). Associations were established using standard genetic models. Genetic risk scores (GRS) [unweighted GRS (unGRS), weighted GRS (wGRS)] were also computed. Correspondingly, the areas under the receiver operating characteristic curves (AUCs) for genetic and non-genetic risk factors were estimated to explore their VT risk predictability in the study population.
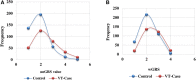
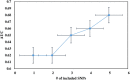
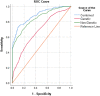
Results: rs6025 was the most prevalent VT risk allele in the Hungarian population. Its risk allele frequency was 3.52-fold higher in the VT group than that in the control group [adjusted odds ratio (AOR) = 3.52, 95% CI: 2.50-4.95]. Using all genetic models, we found that rs6025 and rs2036914 remained significantly associated with VT risk after multiple correction testing was performed. However, rs8176719 remained statistically significant only in the multiplicative (AOR = 1.33, 95% CI: 1.07-1.64) and genotypic models (AOR = 1.77, 95% CI: 1.14-2.73). In addition, rs2066865 lost its significant association with VT risk after multiple correction testing was performed. Conversely, the prothrombin mutation (rs1799963) did not show any significant association. The AUC of Leiden mutation (rs6025) showed better discriminative accuracy than that of other SNPs (AUC = 0.62, 95% CI: 0.57-0.66). The wGRS was a better predictor for VT than the unGRS (AUC = 0.67 vs. 0.65). Furthermore, combining genetic and non-genetic VT risk factors significantly increased the AUC to 0.89 with statistically significant differences (Z = 3.924, p < 0.0001).
Conclusions: Our study revealed that the five strongly associated SNPs combined with non-genetic factors could efficiently predict individual VT risk susceptibility. The combined model was the best predictor of VT risk, so stratifying high-risk individuals based on their genetic profiling and well-known non-modifiable VT risk factors was important for the effective and efficient utilization of VT risk preventive and control measures. Furthermore, we urged further study that compares the VT risk predictability in the Hungarian population using the formerly discovered VT SNPs with the novel strongly associated VT SNPs.
Keywords: Hungarian population; cardiovascular risk (CVD); risk prediction; single nucleotide polymorphisms (SNPs); venous thrombosis.
© 2023 Natae, Merzah, Sándor, Ádány, Bereczky and Fiatal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Higher Prevalence of Venous Thromboembolism in the Hungarian Roma Population Could Be Due to Elevated Genetic Risk and Stronger Gene-Environmental Interactions.Front Cardiovasc Med. 2021 Oct 26;8:647416. doi: 10.3389/fcvm.2021.647416. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34765649 Free PMC article.
-
The Prevalence of the Thrombotic SNPs rs6025, rs1799963, rs2066865, rs2289252 and rs8176719 in Patients with Venous Thromboembolism in the Czech Population.Clin Appl Thromb Hemost. 2025 Jan-Dec;31:10760296251324202. doi: 10.1177/10760296251324202. Epub 2025 Mar 17. Clin Appl Thromb Hemost. 2025. PMID: 40094632 Free PMC article.
-
The incidence of the thrombophilic SNPs rs6025, rs1799963, rs2066865, rs2289252, and rs8176719 in chronic thromboembolic pulmonary hypertension.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241271369. doi: 10.1177/10760296241271369. Clin Appl Thromb Hemost. 2024. PMID: 39150410 Free PMC article.
-
Venous thromboembolism GWAS reported genetic makeup and the hallmarks of cancer: Linkage to ovarian tumour behaviour.Biochim Biophys Acta Rev Cancer. 2020 Jan;1873(1):188331. doi: 10.1016/j.bbcan.2019.188331. Epub 2019 Nov 2. Biochim Biophys Acta Rev Cancer. 2020. PMID: 31689458 Review.
-
Genetics of Venous Thrombosis: update in 2015.Thromb Haemost. 2015 Nov;114(5):910-9. doi: 10.1160/TH15-05-0410. Epub 2015 Sep 10. Thromb Haemost. 2015. PMID: 26354877 Review.
Cited by
-
Impact of Gene-Smoking Interaction on Risk of Atherosclerosis: Molecular Study of Prothrombin (F2) Gene rs1799963 G/A Polymorphism in Atherosclerotic Patients.Cardiovasc Toxicol. 2025 Jun;25(6):867-873. doi: 10.1007/s12012-025-09997-z. Epub 2025 Apr 22. Cardiovasc Toxicol. 2025. PMID: 40261540
References
-
- Natae SF, Kósa Z, Sándor J, Merzah MA, Bereczky Z, Pikó P, et al. The higher prevalence of venous thromboembolism in the Hungarian Roma population could be due to elevated genetic risk and stronger gene-environmental interactions. Front Cardiovasc Med. (2021) 8:647416. 10.3389/fcvm.2021.647416 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

