Mim8, a novel factor VIIIa mimetic bispecific antibody, shows favorable safety and pharmacokinetics in healthy adults
- PMID: 37745159
- PMCID: PMC10514552
- DOI: 10.1016/j.rpth.2023.102181
Mim8, a novel factor VIIIa mimetic bispecific antibody, shows favorable safety and pharmacokinetics in healthy adults
Abstract
Background: Mim8 (denecimig) is a novel activated coagulation factor VIII-mimetic bispecific antibody that assembles with activated coagulation FIX and FX on the platelet membrane surface.
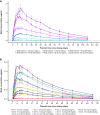
Objectives: The FRONTIER1 (NCT04204408, NN7769-4513) single ascending dose and the 4882 pharmacokinetic (PK) studies (NCT05127473, NN7769-4882) examined the safety, tolerability, PK, and pharmacodynamics (PD) of Mim8 in healthy adult males.
Methods: The FRONTIER1 single ascending dose study consisted of 6 cohorts, each with 6 participants who received a single subcutaneous (s.c.) dose of Mim8 and 2 participants who received a placebo. The 4882 PK study had 11 arms, each with 6 participants who received a single s.c. dose of Mim8. The primary endpoint for both studies was treatment-emergent adverse events. Other safety assessments included relative changes in D-dimer, prothrombin fragments 1 and 2, fibrinogen, and platelets. The PK and PD were assessed using Mim8 plasma concentration and activated partial thromboplastin clotting time and thrombin generation, respectively.
Results: Mim8 was well tolerated, and there were no severe treatment-emergent adverse events. The PK properties of Mim8 in both studies were consistent with dose-proportionality. The terminal half-life of Mim8 after a single dose was approximately 1 month, and maximum plasma concentration was reached after 10 days.
Conclusion: The PK and PD profiles suggest that Mim8 is suitable as a long-acting FVIIIa-mimetic bispecific antibody for hemophilia A prophylaxis.
Keywords: factor VIII; hemophilia A; pharmacokinetics; safety; thrombin.
© 2023 The Author(s).
Figures
References
LinkOut - more resources
Full Text Sources