Long-Acting Injectable Antipsychotic Treatment for Schizophrenia in Asian Population: A Scoping Review
- PMID: 37745189
- PMCID: PMC10516218
- DOI: 10.2147/NDT.S413371
Long-Acting Injectable Antipsychotic Treatment for Schizophrenia in Asian Population: A Scoping Review
Abstract
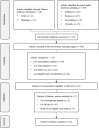
Evidence of comparative benefits of long-acting injectable (LAI) antipsychotics in Asian patients with schizophrenia has been inconsistent. This scoping review aimed to synthesize the current evidence in the past ten years and provide an overview of efficacy, safety, treatment adherence, patient attitudes, and healthcare resource utilization of LAI in this population. A systematic search was conducted with a pre-defined search strategy in six electronic databases including Chinese National Knowledge Infrastructure (CNKI), Wanfang, PubMed, Embase, CINAHL, and PsycArticles. A total of 46 studies were included, including 15 cohort studies, 13 single-arm trials, 10 randomized controlled trials, four mirror-image studies, three cross-sectional studies, and one controlled clinical trial. Paliperidone palmitate once-monthly injection (27/46) and risperidone LAI (14/46) were the most frequently investigated LAIs. Compared with oral antipsychotic medications (OAMs), LAIs demonstrated a lower rate of relapse/hospitalization and comparable improvement in efficacy. Adverse events (AEs) were similar between LAIs and OAMs, although types and incidence varied. Significant reduction in the length of hospitalization and number of outpatient visits/inpatient admission was observed after initiation of LAIs. These findings suggest that LAI demonstrated comparable efficacy and safety among Asian populations with schizophrenia in comparison to OAMs. Better adherence and lower relapse were observed in patients receiving LAIs from published evidence. Future research is warranted to better understand the comprehensive performance of LAI in specific population or context.
Keywords: Asian; long-acting injectable antipsychotics; schizophrenia; scoping review.
© 2023 Ma et al.
Conflict of interest statement
Zhang L, Ye C and Li X are employees of Xi’an Janssen Pharmaceutical Ltd. All other authors have no conflicts of interest to declare in relation to this study.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous