Viral skin diseases in odontocete cetaceans: gross, histopathological, and molecular characterization of selected pathogens
- PMID: 37745220
- PMCID: PMC10514499
- DOI: 10.3389/fvets.2023.1188105
Viral skin diseases in odontocete cetaceans: gross, histopathological, and molecular characterization of selected pathogens
Abstract
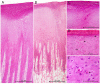
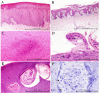
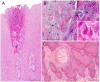
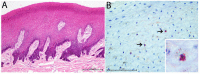
Fifty-five skin lesions from 31 stranded cetaceans along the Canary coasts (2011-2021) were submitted to macroscopic, histological, and molecular analyses to confirm infection by cetacean poxvirus, herpesvirus and cetacean morbillivirus. They were macroscopically categorized into eight categories with respective subcategories according to their color, shape, size, and consistency. Cetacean poxvirus was detected in 54.54% of the skin lesions through real-time and conventional PCRs based on the DNA polymerase gene. Additionally, herpesvirus and morbillivirus were currently detected from 43.63 and 1.82% of the cutaneous lesions, respectively. Coinfection of poxvirus and herpesvirus was detected in nine of them (16.36%), which makes the present study the first to report coinfection by both pathogens in skin lesions in cetaceans. A plausible approach to histopathological characterization of poxvirus-and herpesvirus-positive skin lesions was established. Hyperkeratosis, acanthosis, ballooning degeneration, and intracytoplasmic inclusion bodies in vacuolized keratinocytes through the stratum spinosum were common findings in poxvirus skin lesions. Alphaherpesvirus was associated with a prominent acanthotic epidermis, moderate necrosis, multifocal dyskeratosis, and irregular keratinocytes with both cellular and nuclei pleomorphism. The common histopathological findings of both pathogens were observed in coinfection lesions. However, those associated with herpesvirus were considerably more remarkable. Relationships between molecular and microscopic findings were observed for the lesions that showed tattoo-like and tortuous patterns. Further multidisciplinary diagnostic studies of infected skin lesions are needed to understand the epidemiology of these emerging infectious diseases.
Keywords: cetacean poxvirus; coinfection; herpesvirus; histopathology; molecular diagnosis; morbillivirus; skin lesions.
Copyright © 2023 Segura-Göthlin, Fernández, Arbelo, Andrada Borzollino, Felipe-Jiménez, Colom-Rivero, Fiorito and Sierra.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Molecular identification and microscopic characterization of poxvirus in a Guiana dolphin and a common bottlenose dolphin, Brazil.Dis Aquat Organ. 2018 Sep 27;130(3):177-185. doi: 10.3354/dao03271. Dis Aquat Organ. 2018. PMID: 30259870
-
Molecular characterization of poxviruses associated with tattoo skin lesions in UK cetaceans.PLoS One. 2013 Aug 13;8(8):e71734. doi: 10.1371/journal.pone.0071734. eCollection 2013. PLoS One. 2013. PMID: 23967239 Free PMC article.
-
Alpha- and gammaherpesviruses in stranded striped dolphins (Stenella coeruleoalba) from Spain: first molecular detection of gammaherpesvirus infection in central nervous system of odontocetes.BMC Vet Res. 2020 Aug 12;16(1):288. doi: 10.1186/s12917-020-02511-3. BMC Vet Res. 2020. PMID: 32787898 Free PMC article.
-
A review of virus infections of cataceans and the potential impact of morbilliviruses, poxviruses and papillomaviruses on host population dynamics.Dis Aquat Organ. 1999 Oct 11;38(1):53-65. doi: 10.3354/dao038053. Dis Aquat Organ. 1999. PMID: 10590929 Review.
-
Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors.Dis Aquat Organ. 2009 Sep 23;86(2):143-57. doi: 10.3354/dao02101. Dis Aquat Organ. 2009. PMID: 19902843 Review.
Cited by
-
Physical Measures of Welfare in Fin (Balaenoptera physalus) and Humpback Whales (Megaptera novangliae) Found in an Anthropized Environment: Validation of a First Animal-Based Indicator in Mysticetes.Animals (Basel). 2024 Dec 5;14(23):3519. doi: 10.3390/ani14233519. Animals (Basel). 2024. PMID: 39682484 Free PMC article.
-
Novel Gammaherpesvirus Infections in Narrow-Ridged Finless Porpoise (Neophocaena asiaeorientalis) and False Killer Whales (Pseudorca crassidens) in the Republic of Korea.Viruses. 2024 Jul 31;16(8):1234. doi: 10.3390/v16081234. Viruses. 2024. PMID: 39205209 Free PMC article.
References
-
- Wells R, Rhinehart H, Hansen L, Sweeney J, Townsend F, Stone R, et al. . Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth. (2004) 1:246–54. doi: 10.1007/s10393-004-0094-6 - DOI
-
- Bossart GD, Duignan PJ. Emerging viruses in marine mammals. CAB Rev. (2018) 13:1–17. doi: 10.1079/PAVSNNR.2019.13052 - DOI
-
- van Bressem MFE, Flach M-F, Reyes L, Echegaray JC, Santos M, Viddi F, et al. . Epidemiological characteristics of skin disorders in cetaceans from South American waters. LAJAM. (2015) 10:20–32. doi: 10.5597/lajam190 - DOI
LinkOut - more resources
Full Text Sources

