Grainyhead-like 2 is required for morphological integrity of mouse embryonic stem cells and orderly formation of inner ear-like organoids
- PMID: 37745294
- PMCID: PMC10513505
- DOI: 10.3389/fcell.2023.1112069
Grainyhead-like 2 is required for morphological integrity of mouse embryonic stem cells and orderly formation of inner ear-like organoids
Abstract
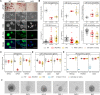
Mutations in the transcription factor gene grainyhead-like 2 (GRHL2) are associated with progressive non-syndromic sensorineural deafness autosomal dominant type 28 (DFNA28) in humans. Since complete loss of Grhl2 is lethal in mouse embryos, we studied its role during inner ear pathology and hearing loss in vitro. To this end, we generated different homozygous deletions to knockout Grhl2 in mouse embryonic stem cells (Grhl2-KO ESCs), including some mimicking naturally occurring truncations in the dimerisation domain related to human DFNA28. Under naïve culture conditions, Grhl2-KO cells in suspension were more heterogenous in size and larger than wild-type controls. Adherent Grhl2-KO cells were also larger, with a less uniform shape, flattened, less circular morphology, forming loose monolayer colonies with poorly defined edges. These changes correlated with lower expression of epithelial cadherin Cdh1 but no changes in tight junction markers (Ocln, Tjp2) or other Grhl isoforms (Grhl1, Grhl3). Clonogenicity from single cells, proliferation rates of cell populations and proliferation markers were reduced in Grhl2-KO ESCs. We next induced stepwise directed differentiation of Grhl2-KO ESCs along an otic pathway, giving rise to three-dimensional inner ear-like organoids (IELOs). Quantitative morphometry revealed that Grhl2-KO cells initially formed larger IELOs with a less compacted structure, more eccentric shape and increased surface area. These morphological changes persisted for up to one week. They were partially rescued by forced cell aggregation and fully restored by stably overexpressing exogenous Grhl2 in Grhl2-KO ESCs, indicating that Grhl2 alters cell-cell interactions. On day 8, aggregates were transferred into minimal maturation medium to allow self-guided organogenesis for another two weeks. During this period, Grhl2-KO cells and wild-type controls developed similarly, expressing neural, neuronal and sensory hair cell markers, while maintaining their initial differences in size and shape. In summary, Grhl2 is required for morphological maintenance of ESCs and orderly formation of IELOs, consistent with an essential role in organising epithelial integrity during inner ear development. Our findings validate quantitative morphometry as a useful, non-invasive screening method for molecular phenotyping of candidate mutations during organoid development.
Keywords: autosomal-dominant hearing loss; cadherin; cell adhesion; embryonic stem cells; grainyhead; inner ear; organoids; quantitative morphometry.
Copyright © 2023 Forrester-Gauntlett, Peters and Oback.
Conflict of interest statement
BF-G and BO were employed by AgResearch Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

