Extracellular vesicles promote activation of pro-inflammatory cancer-associated fibroblasts in oral cancer
- PMID: 37745296
- PMCID: PMC10513103
- DOI: 10.3389/fcell.2023.1240159
Extracellular vesicles promote activation of pro-inflammatory cancer-associated fibroblasts in oral cancer
Abstract
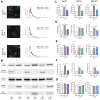
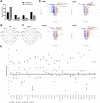
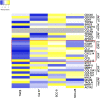
Introduction: Oral squamous cell carcinoma (OSCC) is the most common form of head and neck cancer and has a survival rate of ∼50% over 5 years. New treatment strategies are sorely needed to improve survival rates-and a better understanding of the mechanisms underlying tumorigenesis is needed to develop these strategies. The role of the tumor microenvironment (TME) has increasingly been identified as crucial in tumor progression and metastasis. One of the main constituents of the TME, cancer-associated fibroblasts (CAFs), plays a key role in influencing the biological behavior of tumors. Multiple mechanisms contribute to CAF activation, such as TGFβ signaling, but the role of extracellular vesicles (EVs) in CAF activation in OSCC is poorly understood. Assessing the impact of oral cancer-derived EVs on CAF activation will help to better illuminate OSCC pathophysiology and may drive development of novel treatments options. Methods: EVs were isolated from OSCC cell lines (Cal 27, SCC-9, SCC-25) using differential centrifugation. Nanoparticle tracking analysis was used for EV characterization, and Western blot to confirm the presence of EV protein markers. Oral fibroblasts were co-cultured with enriched EVs, TGFβ, or PBS over 72 h to assess activation. Flow cytometry was used to evaluate CAF markers. RNA collected from fibroblasts was extracted and the transcriptome was sequenced. Conditioned media from the co-cultures was evaluated with cytokine array profiling. Results: OSCC-derived EVs can activate oral fibroblasts into CAFs that are different from those activated by TGFβ, suggesting different mechanisms of activation and different functional properties. Gene set enrichment analysis showed several upregulated inflammatory pathways in those CAFs exposed to OSCC-derived EVs. Marker genes for inflammatory CAF subtypes were also upregulated, but not in CAFs activated by TGFβ. Finally, cytokine array analysis on secreted proteins revealed elevated levels of several pro-inflammatory cytokines from EV-activated CAFs, for instance IL-8 and CXCL5. Discussion: Our results reveal the ability of OSCC-derived EVs to activate fibroblasts into CAFs. These CAFs seem to have unique properties, differing from TGFβ-activated CAFs. Gaining an understanding of the interplay between EVs and stromal cells such as CAFs could lead to further insights into OSCC tumorigenesis and potential novel therapeutics.
Keywords: cancer-associated fibroblast; extracellular vesicles; inflammation; oral squamous cell carcinoma; tumor microenvironment; tumorigenesis.
Copyright © 2023 Arebro, Towle, Lee, Bennewith and Garnis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alfaro C., Teijeira A., Oñate C., Pérez G., Sanmamed M. F., Andueza M. P., et al. (2016). Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22 (15), 3924–3936. 10.1158/1078-0432.ccr-15-2463 - DOI - PubMed
-
- Biffi G., Oni T. E., Spielman B., Hao Y., Elyada E., Park Y., et al. (2019). IL1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9 (2), 282–301. 10.1158/2159-8290.cd-18-0710 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

