This is a preprint.
Clinicopathologic Dissociation: Robust Lafora Body Accumulation in Malin KO Mice Without Observable Changes in Home-cage Behavior
- PMID: 37745312
- PMCID: PMC10515855
- DOI: 10.1101/2023.09.11.557226
Clinicopathologic Dissociation: Robust Lafora Body Accumulation in Malin KO Mice Without Observable Changes in Home-cage Behavior
Update in
-
Clinicopathologic Dissociation: Robust Lafora Body Accumulation in Malin KO Mice Without Observable Changes in Home-Cage Behavior.J Comp Neurol. 2024 Jul;532(7):e25660. doi: 10.1002/cne.25660. J Comp Neurol. 2024. PMID: 39039998 Free PMC article.
Abstract
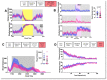
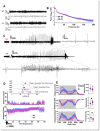
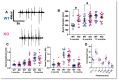
Lafora Disease (LD) is a syndrome of progressive myoclonic epilepsy and cumulative neurocognitive deterioration caused by recessively inherited genetic lesions of EPM2A (laforin) or NHLRC1 (malin). Neuropsychiatric symptomatology in LD is thought to be directly downstream of neuronal and astrocytic polyglucosan aggregates, termed Lafora bodies (LBs), which faithfully accumulate in an age-dependent manner in all mouse models of LD. In this study, we applied home-cage monitoring to examine the extent of neurobehavioral deterioration in a model of malin-deficient LD, as a means to identify robust preclinical endpoints that may guide the selection of novel genetic treatments. At 6 weeks, ~6-7 months and ~12 months of age, malin deficient mice ("KO") and wild type (WT) littermates underwent a standardized home-cage behavioral assessment designed to non-obtrusively appraise features of rest/arousal, consumptive behaviors, risk aversion and voluntary wheel-running. At all timepoints, and over a range of metrics that we report transparently, WT and KO mice were essentially indistinguishable. In contrast, within WT mice compared across timepoints, we identified age-related nocturnal hypoactivity, diminished sucrose preference and reduced wheel-running. Neuropathological examinations in subsets of the same mice revealed expected age dependent LB accumulation, gliosis and microglial activation in cortical and subcortical brain regions. At 12 months of age, despite the burden of neocortical LBs, we did not identify spontaneous seizures during an electroencephalographic (EEG) survey, and KO and WT mice exhibited similar spectral EEG features. Using an in vitro assay of neocortical function, paroxysmal increases in network activity (UP states) in KO slices were more prolonged at 3 and 6 months of age, but were similar to WT at 12 months. KO mice displayed a distinct response to pentylenetetrazole, with a greater incidence of clonic seizures and a more pronounced post-ictal suppression of movement, feeding and drinking behavior. Together, these results highlight a stark clinicopathologic dissociation in a mouse model of LD, where LBs accrue substantially without clinically meaningful changes in overall wellbeing. Our findings allude to a delay between LB accumulation and neurobehavioral decline: one that may provide a window for treatment, and whose precise duration may be difficult to ascertain within the typical lifespan of a laboratory mouse.
Keywords: Lafora body disease; astrogliosis; glycogen storage; home-cage behavior; malin; polyglucosan.
Conflict of interest statement
Conflict of interest disclosure: The authors have no relevant conflicting interests to disclose.
Figures




References
-
- Singh S, Satishchandra P, Shankar SK, Ganesh S. Lafora disease in the Indian population: EPM2A and NHLRC1 gene mutations and their impact on subcellular localization of laforin and malin. Hum Mutat. 2008;29(6):E1–12. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
