This is a preprint.
A latent cardiomyocyte regeneration potential in human heart disease
- PMID: 37745322
- PMCID: PMC10515906
- DOI: 10.1101/2023.09.14.557681
A latent cardiomyocyte regeneration potential in human heart disease
Update in
-
A Latent Cardiomyocyte Regeneration Potential in Human Heart Disease.Circulation. 2025 Jan 21;151(3):245-256. doi: 10.1161/CIRCULATIONAHA.123.067156. Epub 2024 Nov 21. Circulation. 2025. PMID: 39569515 Free PMC article.
Abstract
Cardiomyocytes in the adult human heart show a regenerative capacity, with an annual renewal rate around 0.5%. Whether this regenerative capacity of human cardiomyocytes is employed in heart failure has been controversial. Using retrospective 14C birth dating we analyzed cardiomyocyte renewal in patients with end-stage heart failure. We show that cardiomyocyte generation is minimal in end-stage heart failure patients at rates 18-50 times lower compared to the healthy heart. However, patients receiving left ventricle support device therapy, who showed significant functional and structural cardiac improvement, had a >6-fold increase in cardiomyocyte renewal relative to the healthy heart. Our findings reveal a substantial cardiomyocyte regeneration potential in human heart disease, which could be exploited therapeutically.
Conflict of interest statement
The authors declare no competing interests.
Figures
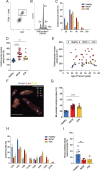

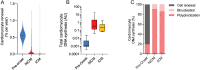


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
