This is a preprint.
Reviving immunogenic cell death upon targeting TACC3 enhances T-DM1 response in HER2-positive breast cancer
- PMID: 37745348
- PMCID: PMC10515808
- DOI: 10.1101/2023.09.12.557273
Reviving immunogenic cell death upon targeting TACC3 enhances T-DM1 response in HER2-positive breast cancer
Update in
-
Targeting TACC3 Induces Immunogenic Cell Death and Enhances T-DM1 Response in HER2-Positive Breast Cancer.Cancer Res. 2024 May 2;84(9):1475-1490. doi: 10.1158/0008-5472.CAN-23-2812. Cancer Res. 2024. PMID: 38319231 Free PMC article.
Abstract
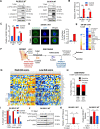
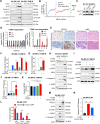
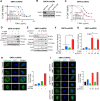
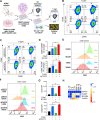
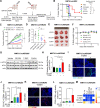
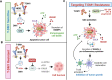
Immunogenic cell death (ICD), an immune-priming form of cell death, has been shown to be induced by several different anti-cancer therapies. Despite being the first and one of the most successful antibody-drug conjugates (ADCs) approved for refractory HER2-positive breast cancer, little is known if response and resistance to trastuzumab emtansine (T-DM1) involves ICD modulation that can be leveraged to enhance T-DM1 response. Here, we report that T-DM1 induces spindle assembly checkpoint (SAC)-dependent ICD in sensitive cells by inducing eIF2α phosphorylation, surface exposure of calreticulin, ATP and HMGB1 release, and secretion of ICD-related cytokines, all of which are lost in resistance. Accordingly, an ICD-related gene signature correlates with clinical response to T-DM1-containing therapy. We found that transforming acidic coiled-coil containing 3 (TACC3) is overexpressed in T-DM1 resistant cells, and that T-DM1 responsive patients have reduced TACC3 protein while the non-responders exhibited increased TACC3 expression during T-DM1 treatment. Notably, genetic or pharmacological inhibition of TACC3 revives T-DM1-induced SAC activation and induction of ICD markers in vitro. Finally, TACC3 inhibition elicits ICD in vivo shown by vaccination assay, and it potentiates T-DM1 by inducing dendritic cell (DC) maturation and enhancing infiltration of cytotoxic T cells in the human HER2-overexpressing MMTV.f.huHER2#5 (Fo5) transgenic model. Together, our results show that ICD is a key mechanism of action of T-DM1 which is lost in resistance, and that targeting TACC3 restores T-DM1-mediated ICD and overcomes resistance.
Keywords: Immunologic cell death; T-DM1; TACC3; antibody drug conjugate; breast cancer; drug resistance; mitotic arrest.
Conflict of interest statement
Declaration of Interest O. Sahin, B.C. and E.B. are the co-founders of OncoCube Therapeutics LLC. O. Sahin is the president of LoxiGen, Inc. The other authors declare no potential conflicts of interest.
Figures
References
-
- Galluzzi L., Buque A., Kepp O., Zitvogel L.,Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017;17:97–111. - PubMed
-
- Kroemer G., Galassi C., Zitvogel L.,Galluzzi L. Immunogenic cell stress and death. Nat Immunol 2022;23:487–500. - PubMed
-
- Galluzzi L., Humeau J., Buque A., Zitvogel L.,Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol 2020;17:725–741. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
