This is a preprint.
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology
- PMID: 37745383
- PMCID: PMC10515813
- DOI: 10.1101/2023.09.11.557277
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology
Update in
-
Interferon lambda restricts herpes simplex virus skin disease by suppressing neutrophil-mediated pathology.mBio. 2024 Apr 10;15(4):e0262323. doi: 10.1128/mbio.02623-23. Epub 2024 Mar 1. mBio. 2024. PMID: 38426749 Free PMC article.
Abstract
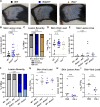
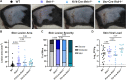
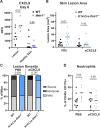
Type III interferons (IFN-λ) are antiviral and immunomodulatory cytokines that have been best characterized in respiratory and gastrointestinal infections, but the effects of IFN-λ against skin infections have not been extensively investigated. We sought to define the skin-specific effects of IFN-λ against the highly prevalent human pathogen herpes simplex virus (HSV). We infected mice lacking the IFN-λ receptor (Ifnlr1-/-), both the IFN-λ and the IFN-αβ receptor (Ifnar1-/- Ifnlr1-/-), or IFN-λ cytokines (Ifnl2/3-/-) and found that IFN-λ restricts the severity of HSV-1 and HSV-2 skin lesions, independent of a direct effect on viral load. Using conditional knockout mice, we found that IFN-λ signaling in both keratinocytes and neutrophils was necessary to control HSV-1 skin lesion severity, and that IFN-λ signaling in keratinocytes suppressed CXCL9-mediated neutrophil recruitment to the skin. Furthermore, depleting neutrophils or blocking CXCL9 protected against severe HSV-1 skin lesions in Ifnlr1-/- mice. Altogether, our results suggest that IFN-λ plays an immunomodulatory role in the skin that restricts neutrophil-mediated pathology during HSV infection, and suggest potential applications for IFN-λ in treating viral skin infections.
Figures






References
-
- Ank N., Iversen M. B., Bartholdy C., Staeheli P., Hartmann R., Jensen U. B., Dagnaes-Hansen F., Thomsen A. R., Chen Z., Haugen H., Klucher K., & Paludan S. R. (2008). An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. Journal of Immunology (Baltimore, Md.: 1950), 180(4), 2474–2485. 10.4049/jimmunol.180.4.2474 - DOI - PubMed
-
- Baldridge M. T., Lee S., Brown J. J., McAllister N., Urbanek K., Dermody T. S., Nice T. J., & Virgin H. W. (2017). Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus. Journal of Virology, 91(7), e02079–16. 10.1128/JVI.02079-16 - DOI - PMC - PubMed
-
- Blazek K., Eames H. L., Weiss M., Byrne A. J., Perocheau D., Pease J. E., Doyle S., McCann F., Williams R. O., & Udalova I. A. (2015). IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. The Journal of Experimental Medicine, 212(6), 845–853. 10.1084/jem.20140995 - DOI - PMC - PubMed
-
- Boff D., Crijns H., Janssens R., Vanheule V., Menezes G. B., Macari S., Silva T. A., Amaral F. A., & Proost P. (2018). The chemokine fragment CXCL9(74–103) diminishes neutrophil recruitment and joint inflammation in antigen-induced arthritis. Journal of Leukocyte Biology, 104(2), 413–422. 10.1002/JLB.3MA1217-502R - DOI - PubMed
-
- Boff D., Russo R. C., Crijns H., de Oliveira V. L. S., Mattos M. S., Marques P. E., Menezes G. B., Vieira A. T., Teixeira M. M., Proost P., & Amaral F. A. (2022). The Therapeutic Treatment with the GAG-Binding Chemokine Fragment CXCL9(74–103) Attenuates Neutrophilic Inflammation and Lung Dysfunction during Klebsiella pneumoniae Infection in Mice. International Journal of Molecular Sciences, 23(11), 6246. 10.3390/ijms23116246 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
