This is a preprint.
The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation
- PMID: 37745436
- PMCID: PMC10515745
- DOI: 10.1101/2023.06.21.545778
The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation
Update in
-
The neural basis of resting-state fMRI functional connectivity in fronto-limbic circuits revealed by chemogenetic manipulation.Nat Commun. 2024 May 31;15(1):4669. doi: 10.1038/s41467-024-49140-0. Nat Commun. 2024. PMID: 38821963 Free PMC article.
Abstract
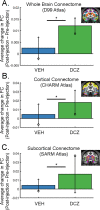
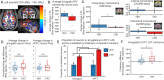
Measures of fMRI resting-state functional connectivity (rs-FC) are an essential tool for basic and clinical investigations of fronto-limbic circuits. Understanding the relationship between rs-FC and neural activity in these circuits is therefore vital. Here we introduced inhibitory designer receptors exclusively activated by designer drugs (DREADDs) into the macaque amygdala and activated them with a highly selective and potent DREADD agonist, deschloroclozapine. We evaluated the causal effect of activating the DREADD receptors on rs-FC and neural activity within circuits connecting amygdala and frontal cortex. Interestingly, activating the inhibitory DREADD increased rs-FC between amygdala and ventrolateral prefrontal cortex. Neurophysiological recordings revealed that the DREADD-induced increase in fMRI rs-FC was associated with increased local field potential coherency in the alpha band (6.5-14.5Hz) between amygdala and ventrolateral prefrontal cortex. Thus, our multi-disciplinary approach reveals the specific signature of neuronal activity that underlies rs-FC in fronto-limbic circuits.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures




References
-
- Aggleton JP, Mishkin M (1984) Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol 222:56–68. - PubMed
-
- Amaral D, Price J, Pitkanen A, Carmichael T (1992) Anatomical organization of the primate amygdaloid complex. In: The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. (Aggleton J, ed), pp 1–66. New York, NY, US, NY, US: Wiley-Liss.
-
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104:5163–5168 Available at: http://www.ncbi.nlm.nih.gov/pubmed/17360345 [Accessed April 9, 2018]. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
