This is a preprint.
Notch3 deletion regulates HIV-1 gene expression and systemic inflammation to ameliorate chronic kidney disease
- PMID: 37745500
- PMCID: PMC10515825
- DOI: 10.1101/2023.09.12.557484
Notch3 deletion regulates HIV-1 gene expression and systemic inflammation to ameliorate chronic kidney disease
Update in
-
Notch3 deletion regulates HIV-1 gene expression and systemic inflammation to ameliorate chronic kidney disease.Dis Model Mech. 2025 Feb 1;18(2):DMM052056. doi: 10.1242/dmm.052056. Epub 2025 Feb 25. Dis Model Mech. 2025. PMID: 39910908 Free PMC article.
Abstract
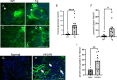
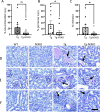
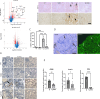
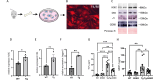
Antiretroviral therapy (ART) has decreased HIV-1 associated morbidity. However, despite ART, immune cells remain latently infected and slowly release viral proteins, leading to chronic inflammation and HIV-1 associated comorbidities. New strategies are needed to target viral proteins and inflammation. We found activation of Notch3 in several renal cells of the HIV-1 mouse model (HIV-Tg26) and in patients with HIV associated Nephropathy. We hypothesized that targeting Notch3 activation constitutes an effective therapy for HIV-related chronic kidney diseases (HIV-CKD). We generated HIV-Tg26 mice with Notch3 knocked out (Tg-N3KO). Compared to HIV-Tg26 mice at 3 months, HIV-Tg-N3KO mice showed a marked reduction in renal injury, skin lesions and mortality rate. Bulk RNA sequencing revealed that N3KO not only reduced renal infiltrating cells but significantly reduced the expression of HIV genes. Moreover, Notch3 activated the HIV- promoter and induction of HIV-1 resulted in increased Notch3 activation indicating a feedback mechanism. Further, bone marrow derived macrophages (BMDMs) from HIV-Tg26 mice showed activation of Notch3 indicating systemic effects. Consistent with that, systemic levels of TNF-α, MCP-1 and other inflammatory chemokines and cytokines were reduced in Tg-N3KO mice. Thus, Notch3 inhibition/deletion has a dual therapeutic effect in HIV-CKD and may extend to other HIV-related pathologies.
Conflict of interest statement
Conflict of interest statement: The authors have declared that no conflict of interest exists.
Figures


References
-
- Gonzales CB, Smith S, Galvan A, Mabry J. The differences between providing oral health care to HIV-infected children and HIV-infected adults: a general dentist’s guide. Gen Dent. Sep-Oct 2010;58(5):424–32; quiz 733–4. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
