This is a preprint.
The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A
- PMID: 37745542
- PMCID: PMC10515842
- DOI: 10.1101/2023.09.12.557419
The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A
Update in
-
The αC-β4 loop controls the allosteric cooperativity between nucleotide and substrate in the catalytic subunit of protein kinase A.Elife. 2024 Jun 24;12:RP91506. doi: 10.7554/eLife.91506. Elife. 2024. PMID: 38913408 Free PMC article.
Abstract
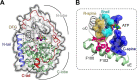
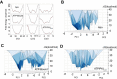
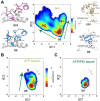
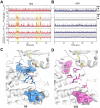
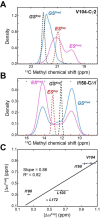
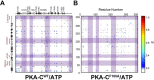
Allosteric cooperativity between ATP and substrates is a prominent characteristic of the cAMP-dependent catalytic (C) subunit of protein kinase A (PKA). Not only this long-range synergistic action is involved in substrate recognition and fidelity, but it is likely to regulate PKA association with regulatory subunits and other binding partners. To date, a complete understanding of the molecular determinants for this intramolecular mechanism is still lacking. Here, we used an integrated NMR-restrained molecular dynamics simulations and a Markov Model to characterize the free energy landscape and conformational transitions of the catalytic subunit of protein kinase A (PKA-C). We found that the apo-enzyme populates a broad free energy basin featuring a conformational ensemble of the active state of PKA-C (ground state) and other basins with lower populations (excited states). The first excited state corresponds to a previously characterized inactive state of PKA-C with the αC helix swinging outward. The second excited state displays a disrupted hydrophobic packing around the regulatory (R) spine, with a flipped configuration of the F100 and F102 residues at the tip of the αC-β4 loop. To experimentally validate the second excited state, we mutated F100 into alanine and used NMR spectroscopy to characterize the binding thermodynamics and structural response of ATP and a prototypical peptide substrate. While the activity of PKA-CF100A toward a prototypical peptide substrate is unaltered and the enzyme retains its affinity for ATP and substrate, this mutation rearranges the αC-β4 loop conformation interrupting the allosteric coupling between nucleotide and substrate. The highly conserved αC-β4 loop emerges as a pivotal element able to modulate the synergistic binding between nucleotide and substrate and may affect PKA signalosome. These results may explain how insertion mutations within this motif affect drug sensitivity in other homologous kinases.
Keywords: Protein Kinases; allosteric mutations; binding cooperativity; cAMP-dependent protein kinase A.
Conflict of interest statement
Competing interest The authors declare no financial and non-financial competing interests.
Figures

References
-
- Manning G., Whyte D.B., Martinez R., Hunter T. & Sudarsanam S. The Protein Kinase Complement of the Human Genome. Science 298, 1912–1934 (2002). - PubMed
-
- Knighton D.R. et al. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 407–14 (1991). - PubMed
-
- Knighton D.R. et al. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science 253, 414–20 (1991). - PubMed
-
- Walsh D.A., Perkins J.P. & Krebs E.G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem 243, 3763–5 (1968). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
