Early Detection of Risk of Neo-Sinus Blood Stasis Post-Transcatheter Aortic Valve Replacement Using Personalized Hemodynamic Analysis
- PMID: 37745677
- PMCID: PMC10512011
- DOI: 10.1016/j.shj.2023.100180
Early Detection of Risk of Neo-Sinus Blood Stasis Post-Transcatheter Aortic Valve Replacement Using Personalized Hemodynamic Analysis
Abstract
Background: Despite the demonstrated benefits of transcatheter aortic valve replacement (TAVR), subclinical leaflet thrombosis and hypoattenuated leaflet thickening are commonly seen as initial indications of decreased valve durability and augmented risk of transient ischemic attack.
Methods: We developed a multiscale patient-specific computational framework to quantify metrics of global circulatory function, metrics of global cardiac function, and local cardiac fluid dynamics of the aortic root and coronary arteries.
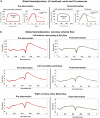
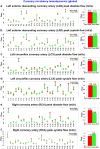
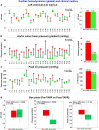
Results: Based on our findings, TAVR might be associated with a high risk of blood stagnation in the neo-sinus region due to the lack of sufficient blood flow washout during the diastole phase (e.g., maximum blood stasis volume increased by 13, 8, and 2.7 fold in the left coronary cusp, right coronary cusp, and noncoronary cusp, respectively [N = 26]). Moreover, in some patients, TAVR might not be associated with left ventricle load relief (e.g., left ventricle load reduced only by 1.2 % [N = 26]) and diastolic coronary flow improvement (e.g., maximum coronary flow reduced by 4.94%, 15.05%, and 23.59% in the left anterior descending, left circumflex coronary artery, and right coronary artery, respectively, [N = 26]).
Conclusions: The transvalvular pressure gradient amelioration after TAVR might not translate into adequate sinus blood washout, optimal coronary flow, and reduced cardiac stress. Noninvasive personalized computational modeling can facilitate the determination of the most effective revascularization strategy pre-TAVR and monitor leaflet thrombosis and coronary plaque progression post-TAVR.
Keywords: Cardiac fluid dynamics; Coronary hemodynamics; Global hemodynamics; Patient-specific lumped parameter model; Transcatheter aortic valve replacement; Valve thrombosis local fluid dynamics.
© 2023 The Authors.
Conflict of interest statement
The authors report no conflict of interest.
Figures





References
-
- Abdul-Jawad Altisent O., Ferreira-Gonzalez I., Marsal J.R., et al. Neurological damage after transcatheter aortic valve implantation compared with surgical aortic valve replacement in intermediate risk patients. Clin Res Cardiol. 2016;105:508–517. - PubMed
-
- Athappan G., Gajulapalli R.D., Sengodan P., et al. Influence of transcatheter aortic valve replacement strategy and valve design on stroke after transcatheter aortic valve replacement: a meta-analysis and systematic review of literature. J Am Coll Cardiol. 2014;63:2101–2110. - PubMed
-
- Bogyi M., Schernthaner R.E., Loewe C., et al. Subclinical leaflet thrombosis after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2021;14:2643–2656. - PubMed
-
- Karády J., Apor A., Nagy A.I., et al. Quantification of hypo-attenuated leaflet thickening after transcatheter aortic valve implantation: clinical relevance of hypo-attenuated leaflet thickening volume. Eur Heart J Cardiovasc Imaging. 2020;21:1395–1404. - PubMed
LinkOut - more resources
Full Text Sources

