Evolutionary conservation of Zinc-Activated Channel (ZAC) functionality in mammals: a range of mammalian ZACs assemble into cell surface-expressed functional receptors
- PMID: 37745686
- PMCID: PMC10513076
- DOI: 10.3389/fmolb.2023.1265429
Evolutionary conservation of Zinc-Activated Channel (ZAC) functionality in mammals: a range of mammalian ZACs assemble into cell surface-expressed functional receptors
Abstract
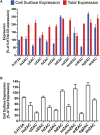
In contrast to the other pentameric ligand-gated ion channels in the Cys-loop receptor superfamily, the ZACN gene encoding for the Zinc-Activated Channel (ZAC) is exclusively found in the mammalian genome. Human ZAC assembles into homomeric cation-selective channels gated by Zn2+, Cu2+ and H+, but the function of the receptor in human physiology is presently poorly understood. In this study, the degree of evolutionary conservation of a functional ZAC in mammals was probed by investigating the abilities of a selection of ZACs from 10 other mammalian species than human to be expressed at the protein level and assemble into cell surface-expressed functional receptors in mammalian cells and in Xenopus oocytes. In an enzyme-linked immunosorbent assay, transient transfections of tsA201 cells with cDNAs of hemagglutinin (HA)-epitope-tagged versions of these 10 ZACs resulted in robust total expression and cell surface expression levels of all proteins. Moreover, injection of cRNAs for 6 of these ZACs in oocytes resulted in the formation of functional receptors in two-electrode voltage-clamp recordings. The ZACs exhibited robust current amplitudes in response to Zn2+ (10 mM) and H+ (pH 4.0), and the concentration-response relationships displayed by Zn2+ at these channels were largely comparable to that at human ZAC. In conclusion, the findings suggest that the functionality of ZAC at the molecular level may be conserved throughout mammalian species, and that the channel thus may govern physiological functions in mammals, including humans.
Keywords: Zinc-Activated Channel (ZAC); evolutionary conservation; mammalian; pentameric ligand-gated ion channel (pLGIC); proton; two-electrode voltage-clamp electrophysiology (TEVC); zinc.
Copyright © 2023 Jensen.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Bloomenthal A. B., Goldwater E., Pritchett D. B., Harrison N. L. (1994). Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ . Mol. Pharmacol. 46, 1156–1159. - PubMed
LinkOut - more resources
Full Text Sources

