Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: role of transcriptional and post-transcriptional events
- PMID: 37745689
- PMCID: PMC10511911
- DOI: 10.3389/fmolb.2023.1257859
Enthralling genetic regulatory mechanisms meddling insecticide resistance development in insects: role of transcriptional and post-transcriptional events
Abstract
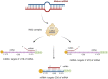
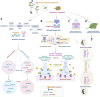
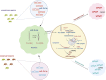
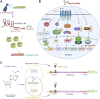
Insecticide resistance in insects severely threatens both human health and agriculture, making insecticides less compelling and valuable, leading to frequent pest management failures, rising input costs, lowering crop yields, and disastrous public health. Insecticide resistance results from multiple factors, mainly indiscriminate insecticide usage and mounted selection pressure on insect populations. Insects respond to insecticide stress at the cellular level by modest yet significant genetic propagations. Transcriptional, co-transcriptional, and post-transcriptional regulatory signals of cells in organisms regulate the intricate processes in gene expressions churning the genetic information in transcriptional units into proteins and non-coding transcripts. Upregulation of detoxification enzymes, notably cytochrome P450s (CYPs), glutathione S-transferases (GSTs), esterases [carboxyl choline esterase (CCE), carboxyl esterase (CarE)] and ATP Binding Cassettes (ABC) at the transcriptional level, modification of target sites, decreased penetration, or higher excretion of insecticides are the noted insect physiological responses. The transcriptional regulatory pathways such as AhR/ARNT, Nuclear receptors, CncC/Keap1, MAPK/CREB, and GPCR/cAMP/PKA were found to regulate the detoxification genes at the transcriptional level. Post-transcriptional changes of non-coding RNAs (ncRNAs) such as microRNAs (miRNA), long non-coding RNAs (lncRNA), and epitranscriptomics, including RNA methylation, are reported in resistant insects. Additionally, genetic modifications such as mutations in the target sites and copy number variations (CNV) are also influencing insecticide resistance. Therefore, these cellular intricacies may decrease insecticide sensitivity, altering the concentrations or activities of proteins involved in insecticide interactions or detoxification. The cellular episodes at the transcriptional and post-transcriptional levels pertinent to insecticide resistance responses in insects are extensively covered in this review. An overview of molecular mechanisms underlying these biological rhythms allows for developing alternative pest control methods to focus on insect vulnerabilities, employing reverse genetics approaches like RNA interference (RNAi) technology to silence particular resistance-related genes for sustained insect management.
Keywords: RNA methylation; detoxification enzymes; insecticide resistance; insects; ncRNAs; pathways.
Copyright © 2023 Muthu Lakshmi Bavithra, Murugan, Pavithran and Naveena.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Insights into the role of non-coding RNAs in the development of insecticide resistance in insects.Front Genet. 2024 Jul 5;15:1429411. doi: 10.3389/fgene.2024.1429411. eCollection 2024. Front Genet. 2024. PMID: 39036703 Free PMC article. Review.
-
Transcription factors CncC/Maf and AhR/ARNT coordinately regulate the expression of multiple GSTs conferring resistance to chlorpyrifos and cypermethrin in Spodoptera exigua.Pest Manag Sci. 2019 Jul;75(7):2009-2019. doi: 10.1002/ps.5316. Epub 2019 Feb 6. Pest Manag Sci. 2019. PMID: 30610747
-
A near-chromosome level genome assembly of the European hoverfly, Sphaerophoria rueppellii (Diptera: Syrphidae), provides comparative insights into insecticide resistance-related gene family evolution.BMC Genomics. 2022 Mar 12;23(1):198. doi: 10.1186/s12864-022-08436-5. BMC Genomics. 2022. PMID: 35279098 Free PMC article.
-
RNA interference: Applications and advances in insect toxicology and insect pest management.Pestic Biochem Physiol. 2015 May;120:109-17. doi: 10.1016/j.pestbp.2015.01.002. Epub 2015 Jan 9. Pestic Biochem Physiol. 2015. PMID: 25987228 Review.
-
Cap 'n' collar C regulates genes responsible for imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata.Insect Biochem Mol Biol. 2018 Aug;99:54-62. doi: 10.1016/j.ibmb.2018.05.006. Epub 2018 May 28. Insect Biochem Mol Biol. 2018. PMID: 29852222
Cited by
-
Emerging Orchestrator of Ecological Adaptation: m6A Regulation of Post-Transcriptional Mechanisms.Mol Ecol. 2025 Aug;34(15):e17545. doi: 10.1111/mec.17545. Epub 2024 Oct 5. Mol Ecol. 2025. PMID: 39367666 Free PMC article. Review.
-
MicroRNA Targets PAP1 to Mediate Melanization in Plutella xylostella (Linnaeus) Infected by Metarhizium anisopliae.Int J Mol Sci. 2024 Jan 17;25(2):1140. doi: 10.3390/ijms25021140. Int J Mol Sci. 2024. PMID: 38256210 Free PMC article.
-
Insights into the role of non-coding RNAs in the development of insecticide resistance in insects.Front Genet. 2024 Jul 5;15:1429411. doi: 10.3389/fgene.2024.1429411. eCollection 2024. Front Genet. 2024. PMID: 39036703 Free PMC article. Review.
-
Non-Coding RNAs Potentially Involved in Pyrethroid Resistance of Anopheles funestus Population in Western Kenya.Res Sq [Preprint]. 2024 Feb 29:rs.3.rs-3979432. doi: 10.21203/rs.3.rs-3979432/v1. Res Sq. 2024. Update in: BMC Genomics. 2025 Jan 23;26(1):64. doi: 10.1186/s12864-025-11260-2. PMID: 38464038 Free PMC article. Updated. Preprint.
-
Exploring the Role of mRNA Methylation in Insect Biology and Resistance.Insects. 2025 Apr 28;16(5):463. doi: 10.3390/insects16050463. Insects. 2025. PMID: 40429176 Free PMC article. Review.
References
-
- APRD (2023). Arthropod pesticide resistance database Michigan state university . https://www.pesticideresistance.org/ (Accessed May 5, 2023).
-
- Atsumi S., Miyamoto K., Yamamoto K., Narukawa J., Kawai S., Sezutsu H., et al. (2012). Single amino acid mutation in an ATP-binding cassette transporter gene causes resistance to Bt toxin Cry1Ab in the silkworm, Bombyx mori . Proc. Natl. Acad. Sci. U.S.A. 109, E1591–E1598. 10.1073/pnas.1120698109 - DOI - PMC - PubMed
-
- Banazeer A., Afzal M. B., Hassan S., Ijaz M., Shad S. A., Serrão J. E. (2021). Status of insecticide resistance in Plutella xylostella (Linnaeus) (Lepidoptera: plutellidae) from 1997 to 2019: cross-resistance, genetics, biological costs, underlying mechanisms, and implications for management. Phytoparasitica 50, 465–485. 10.1007/s12600-021-00959-z - DOI
-
- Bao Y. Y., Zhang C. X. (2019). Recent advances in molecular biology research of a rice pest, the brown planthopper. J. Integr. Agric. 18, 716–728. 10.1016/S2095-3119(17)61888-4 - DOI
Publication types
LinkOut - more resources
Full Text Sources

