Exploring Circulating Tumor DNA (CtDNA) and Its Role in Early Detection of Cancer: A Systematic Review
- PMID: 37745752
- PMCID: PMC10516512
- DOI: 10.7759/cureus.45784
Exploring Circulating Tumor DNA (CtDNA) and Its Role in Early Detection of Cancer: A Systematic Review
Abstract
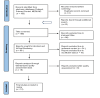
There is a significant increase in the need for an efficient screening method that might identify cancer at an early stage and could improve patients' long-term survival due to the continued rise in cancer incidence and associated mortality. One such effort involved using circulating tumor DNA (ctDNA) as a rescue agent for a non-invasive blood test that may identify many tumors. A tumor marker called ctDNA is created by cells with the same DNA alterations. Due to its shorter half-life, it may be useful for both early cancer detection and real-time monitoring of tumor development, therapeutic response, and tumor outcomes. We obtained 156 papers from PUBMED using the MeSH approach in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) criteria and ten articles from additional online resources. After removing articles with irrelevant titles and screening the abstract and full text of the articles that contained information unrelated to or not specific to the title query using inclusion and exclusion criteria, 18 out of 166 articles were chosen for the quality check. Fourteen medium to high-quality papers were chosen out of the 18 publications to be included in the study design. The reviewed literature showed no significant utility of ctDNA in detecting early-stage tumors of size less than 1 cm diameter. Still, the ideal screening test would require the assay to detect a size <5 mm tumor, which is nearly impossible with the current data. The sensitivity and specificity of the assay ranged from 69% to 98% and 99%, respectively. Furthermore, CancerSEEK achieves tumor origin localization in 83% of cases, while targeted error correction sequencing (TEC-Seq) assays demonstrate a cancer detection rate ranging from 59% to 71%, depending on the type of cancer. However, it could be of great value as a prognostic indicator, and the levels are associated with progression-free survival (PFS) and overall survival (OS) rates, wherein the positive detection of ctDNA is associated with worse OS compared to the tumors detected through standard procedures, with an odds ratio (OS) of 4.83. We conclude that ctDNA could be better applied in cancer patients for prognosis, disease progression monitoring, and treatment outcomes compared to its use in early cancer detection. Due to its specific feature of recognizing the tumor-related mutations, it could be implemented as a supplemental tool to assess the nature of the tumor, grade, and size of the tumor and for predicting the outcomes by pre-operative and post-operative evaluation of the tumor marker, ctDNA, and thereby estimating PFS and OS depending on the level of marker present. A vast amount of research is required in early detection to determine the sensitivity, specificity, false positive rates, and false negative rates in evaluating its true potential as a screening tool. Even if the test could detect the mutations, an extensive workup for the search of tumor is required as the assay could only detect but cannot localize the disease. Establishing the clinical validity and utility of ctDNA is imperative for its implementation in future clinical practice.
Keywords: circulating tumor dna (ctdna); early detection of cancer; liquid biopsy; medical screening; prevention in primary care.
Copyright © 2023, Bittla et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Kocarnik JM, Compton K, Dean FE, et al. JAMA Oncol. 2022;8:420–444. - PMC - PubMed
-
- Late presentation of breast cancer in lower- and middle-income countries. Clegg-Lamptey JA, Vanderpuye V, Dedey F. Curr Breast Cancer Rep. 2019;11:143–151.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources