Safety and efficacy with alemtuzumab over 13 years in relapsing-remitting multiple sclerosis: final results from the open-label TOPAZ study
- PMID: 37745914
- PMCID: PMC10515516
- DOI: 10.1177/17562864231194823
Safety and efficacy with alemtuzumab over 13 years in relapsing-remitting multiple sclerosis: final results from the open-label TOPAZ study
Abstract
Background and objectives: Alemtuzumab demonstrated superior efficacy versus subcutaneous interferon (IFN) beta-1a in participants with relapsing-remitting multiple sclerosis in the 2-year CARE-MS I and II trials. Efficacy was maintained in the 4-year CARE-MS extension, during which alemtuzumab-treated participants ('alemtuzumab-only') could receive additional courses upon disease activity, and IFN-treated participants switched to alemtuzumab ('IFN-alemtuzumab'). Participants who completed the CARE-MS extension could enroll in the open-label TOPAZ study which assessed safety and efficacy for 5-7 years (11-13 years after alemtuzumab/IFN initiation).
Methods: Participants received additional alemtuzumab courses as needed. Assessments included adverse events (AEs; primary outcome), annualized relapse rate (ARR), 6-month confirmed disability worsening [CDW; ⩾1.0-point Expanded Disability Status Scale (EDSS) score increase or ⩾1.5 if baseline EDSS = 0], and 6-month confirmed disease improvement [CDI; >1.0-point EDSS decrease (baseline score ⩾2.0)].
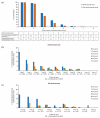
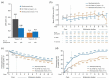
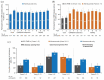
Results: 43.5% of alemtuzumab-only participants from CARE-MS II and 54.2% from CARE-MS I received no additional alemtuzumab courses; 30.0% and 20.9%, respectively, received one additional course (the median). Incidences of AEs, including thyroid AEs and infections, declined over time. The safety profile of alemtuzumab was similar for participants who received zero, one, or two additional courses. For CARE-MS II participants, who had inadequate response to previous treatment, ARR remained low during Years 3-13 for the alemtuzumab-only [0.17; 95% confidence interval (CI) 0.15-0.20] and IFN-alemtuzumab (0.14; 0.11-0.17) groups. At Year 11, the proportions of participants who were either free from CDW or who had CDI were higher in the alemtuzumab-only group (58% and 49%, respectively) than in the IFN-alemtuzumab group (51% and 37%). For CARE-MS I participants, who were previously treatment-naïve, clinical outcomes remained improved, and no between-group differences were apparent.
Conclusion: Safety risks associated with alemtuzumab treatment declined over time. Clinical benefits were maintained up to 11-13 years, and most participants did not require more than one additional course.
Clinicaltrialsgov identifiers: NCT00530348; NCT00548405; NCT00930553; NCT02255656.
Keywords: Alemtuzumab; disease-modifying therapy; follow-up studies; interferon beta-1a; multiple sclerosis; relapsing-remitting.
© The Author(s), 2023.
Conflict of interest statement
AJC reports consulting fees, lecture fees, and institutional grant support from Sanofi up to September 2017. AA reports research and travel grants, honoraria for MS-expert advice and consulting, and/or speaking fees (Biogen, Merck Serono, Novartis, Roche, and Sanofi). AT reports consulting and/or speaking fees and grant/research support (Biogen, EMD Serono, Novartis, Roche, and Sanofi). BAS reports research grant support from AbbVie, Biogen, Bristol Myers Squibb, Greenwich Biosciences, Novartis, and Sanofi; and consulting and/or speaking fees from AbbVie, Alexion, Biogen, Bristol Myers Squibb, Cigna, EMD Serono, Janssen, Genentech, Greenwich Biosciences, Horizon, Novartis, Octave Bioscience, Roche, Sanofi, and TG Therapeutics. CP reports consulting and/or speaking fees, research, and travel grants (Actelion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi, and Teva). COG reports speaking and/or consultancy fees (Bayer, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva). GG reports compensation for serving as a consultant or speaker for or has received research support from AbbVie, Aslan, Atara Bio, Biogen, BMS-Celgene, GlaxoSmithKline, GW Pharma, Janssen/J&J, Japanese Tobacco, Jazz Pharmaceuticals, LifNano, Merck & Co, Merck KGaA/EMD Serono, Moderna, Novartis, Sanofi, Roche/Genentech, and Teva in the past 5 years. GC reports consulting fees (Actelion, Bayer Schering, Merck Serono, Novartis, Sanofi, and Teva) and lecture fees (Bayer Schering, Biogen Dompé, Merck Serono, Novartis, Sanofi, Serono, Symposia International Foundation, and Teva). MS Freedman reports honoraria/consulting fees (Alexion/AstraZeneca, Atara Biotherapeutics, Bayer HealthCare, Beigene, BMS/Celgene, EMD Inc., Hoffman La-Roche, Janssen/J&J, Merck Serono, Quanterix, Novartis, Sanofi, and Teva Canada Innovation); serving as a member of an advisory board, board of directors, or other similar group (Alexion, Atara Biotherapeutics, Bayer HealthCare, Beigene, BMS/Celgene, Celestra, Hoffman La-Roche, Janssen/J&J, McKesson, Merck Serono, Novartis and Sanofi); participation in the speakers bureau (EMD Serono and Sanofi); and grant/research support (Sanofi). TZ reports consulting and/or speaking fees (Almirall, Bayer, Biogen, BMS, Celgene, Merck, Novartis, Roche, Sanofi, Viatris and Teva) and grant/research support (Biogen, BMS, Novartis, Roche, Sanofi, and Teva). DS, AMR, ATW, and MC are employees of Sanofi and may hold shares and/or stock options in the company. XM reports speaking honoraria and travel expenses for scientific meetings, being a steering committee member of clinical trials, or participating in advisory boards of clinical trials in the past 3 years (Actelion, Alexion, Bayer, Biogen, Celgene, EMD Serono, EXCEMED, MedDay, Merck, MSIF, NervGen, NMSS, Novartis, Roche, Sanofi, Teva Pharmaceutical, and TG Therapeutics).
Figures

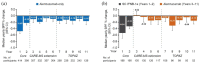
Similar articles
-
Efficacy and Safety of Alemtuzumab Through 9 Years of Follow-up in Patients with Highly Active Disease: Post Hoc Analysis of CARE-MS I and II Patients in the TOPAZ Extension Study.CNS Drugs. 2020 Sep;34(9):973-988. doi: 10.1007/s40263-020-00749-x. CNS Drugs. 2020. PMID: 32710396 Free PMC article. Clinical Trial.
-
Long-term efficacy and safety of alemtuzumab in participants with highly active MS: TOPAZ clinical trial and interim analysis of TREAT-MS real-world study.Ther Adv Neurol Disord. 2025 Feb 10;18:17562864241306575. doi: 10.1177/17562864241306575. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 39935588 Free PMC article.
-
Confirmed 6-Month Disability Improvement and Worsening Correlate with Long-term Disability Outcomes in Alemtuzumab-Treated Patients with Multiple Sclerosis: Post Hoc Analysis of the CARE-MS Studies.Neurol Ther. 2021 Dec;10(2):803-818. doi: 10.1007/s40120-021-00262-3. Epub 2021 Jun 24. Neurol Ther. 2021. PMID: 34165694 Free PMC article.
-
Best Practices for Long-Term Monitoring and Follow-Up of Alemtuzumab-Treated MS Patients in Real-World Clinical Settings.Front Neurol. 2019 Mar 22;10:253. doi: 10.3389/fneur.2019.00253. eCollection 2019. Front Neurol. 2019. PMID: 30967831 Free PMC article. Review.
-
Alemtuzumab: evidence for its potential in relapsing-remitting multiple sclerosis.Drug Des Devel Ther. 2013;7:131-8. doi: 10.2147/DDDT.S32687. Epub 2013 Mar 6. Drug Des Devel Ther. 2013. PMID: 23494602 Free PMC article. Review.
Cited by
-
Alemtuzumab treatment for multiple sclerosis in Austria: An observational long-term outcome study.Ann Clin Transl Neurol. 2024 Jun;11(6):1442-1455. doi: 10.1002/acn3.52056. Epub 2024 May 7. Ann Clin Transl Neurol. 2024. PMID: 38715245 Free PMC article.
-
Optimizing Drug Selection in Children with Multiple Sclerosis: What Do We Know and What Remains Unanswered?Paediatr Drugs. 2025 Mar;27(2):161-179. doi: 10.1007/s40272-024-00675-1. Epub 2024 Dec 26. Paediatr Drugs. 2025. PMID: 39724509 Review.
-
Assessment of the impact of reconstitution therapies-cladribine tablets and alemtuzumab-on the atrophy progression among patients with relapse-remitting multiple sclerosis.Front Neurosci. 2025 Feb 27;19:1531163. doi: 10.3389/fnins.2025.1531163. eCollection 2025. Front Neurosci. 2025. PMID: 40084136 Free PMC article.
-
Practical Recommendations from the Gulf Region on the Therapeutic Use of Cladribine Tablets for the Management of Relapsing Multiple Sclerosis: Impact of the Latest Real-World Evidence on Clinical Practice.Neurol Ther. 2024 Oct;13(5):1321-1335. doi: 10.1007/s40120-024-00650-5. Epub 2024 Aug 3. Neurol Ther. 2024. PMID: 39097537 Free PMC article. Review.
-
The immunological bases of alemtuzumab as induction-therapy in pediatric-onset multiple sclerosis.Front Immunol. 2025 Jan 8;15:1509987. doi: 10.3389/fimmu.2024.1509987. eCollection 2024. Front Immunol. 2025. PMID: 39845956 Free PMC article. Review.
References
-
- Ontaneda D, Tallantyre E, Kalincik T, et al.. Early highly effective versus escalation treatment approaches in relapsing multiple sclerosis. Lancet Neurol 2019; 18: 973–980. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

