The evolving role of data & safety monitoring boards for real-world clinical trials
- PMID: 37745930
- PMCID: PMC10514684
- DOI: 10.1017/cts.2023.582
The evolving role of data & safety monitoring boards for real-world clinical trials
Abstract
Introduction: Clinical trials provide the "gold standard" evidence for advancing the practice of medicine, even as they evolve to integrate real-world data sources. Modern clinical trials are increasingly incorporating real-world data sources - data not intended for research and often collected in free-living contexts. We refer to trials that incorporate real-world data sources as real-world trials. Such trials may have the potential to enhance the generalizability of findings, facilitate pragmatic study designs, and evaluate real-world effectiveness. However, key differences in the design, conduct, and implementation of real-world vs traditional trials have ramifications in data management that can threaten their desired rigor.
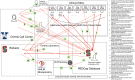
Methods: Three examples of real-world trials that leverage different types of data sources - wearables, medical devices, and electronic health records are described. Key insights applicable to all three trials in their relationship to Data and Safety Monitoring Boards (DSMBs) are derived.
Results: Insight and recommendations are given on four topic areas: A. Charge of the DSMB; B. Composition of the DSMB; C. Pre-launch Activities; and D. Post-launch Activities. We recommend stronger and additional focus on data integrity.
Conclusions: Clinical trials can benefit from incorporating real-world data sources, potentially increasing the generalizability of findings and overall trial scale and efficiency. The data, however, present a level of informatic complexity that relies heavily on a robust data science infrastructure. The nature of monitoring the data and safety must evolve to adapt to new trial scenarios to protect the rigor of clinical trials.
Keywords: Clinical trials; DSMB; RWD; data and safety monitoring board; data integrity; data quality; decentralized; digital; pragmatic; real world data; real-world data; real-world evidence.
© The Author(s) 2023.
Conflict of interest statement
BJB, HH, JDC, JOF, AG, RJ, JL, KOO, MP, KR, HS, MT, & MD have no disclosures. JHC has received research grant support from NIH/National Institute on Drug Abuse Clinical Trials Network (UG1DA015815 - CTN-0136), Stanford Artificial Intelligence in Medicine and Imaging - Human-Centered Artificial Intelligence (AIMI-HAI) Partnership Grant, Doris Duke Charitable Foundation - Covid-19 Fund to Retain Clinical Scientists (20211260), Google, Inc. Research collaboration Co-I to leverage EHR data to predict a range of clinical outcomes, and American Heart Association - Strategically Focused Research Network - Diversity in Clinical Trials. JHC discloses: Co-founder of Reaction Explorer LLC that develops and licenses organic chemistry education software & Paid consulting fees from Sutton Pierce and Younker Hyde MacFarlane PLLC as a medical expert witness. EF is supported in part by AFOSR Grant FA9550-21-1-0397, ONR Grant N00014-22-1-2110, and the Stanford Institute for Human-Centered Artificial Intelligence (HAI). EBF is a Chan Zuckerberg Biohub – San Francisco Investigator. AGo has received research funding from the National Heart, Lung, and Blood Institute; National Institute of Diabetes, Digestive, and Kidney Diseases; and the National Institute on Aging. He has also received research grants through his institution from Novartis, Bristol Myers Squibb, Pfizer, Janssen Research & Development, CSL Behring, iRhythm Technologies, and Amarin Pharmaceuticals. RJ was partially supported by the National Science Foundation under grant 2205084, and by the Stanford Maternal and Child Health Research Institute under the Transdisciplinary Initiatives Program. DMM has had research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust and his institution has had research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. Dr Maahs has consulted for Abbott, Aditxt, the Helmsley Charitable Trust, Lifescan, Mannkind, Sanofi, Novo Nordisk, Eli Lilly, Medtronic, Insulet, Dompe, Biospex, Provention Bio, and Bayer. DS discloses advising Carta Healthcare. MPT has received grants or personal fees from American Heart Association, Apple, Bayer, Bristol Myers Squibb, FDA, Gilead Sciences, Johnson & Johnson, Medtronic Inc., Myokardia, Pfizer, and Sanofi, is a shareholder of AliveCor, Connect America, Evidently, Forward, iRhythm, and PocketRN, and is an employee of iRhythm Technologies, Inc. KWM’s financial disclosures can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey.
Figures



References
-
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. Bmj. 2015;350:h2147. - PubMed
-
- Food and Drug Administration. Guidance for clinical trial sponsors: Establishment and operation of clinical trial data monitoring committees (https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm127073.pdf). Accessed August 15, 2023.
-
- Evans SR. Independent oversight of clinical trials through data and safety monitoring boards. NEJM Evid. 2022;1(1):EVIDctw2100005. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
