Cell-mediated and Neutralizing Antibody Responses to the SARS-CoV-2 Omicron BA.4/BA.5-adapted Bivalent Vaccine Booster in Kidney and Liver Transplant Recipients
- PMID: 37745949
- PMCID: PMC10513127
- DOI: 10.1097/TXD.0000000000001536
Cell-mediated and Neutralizing Antibody Responses to the SARS-CoV-2 Omicron BA.4/BA.5-adapted Bivalent Vaccine Booster in Kidney and Liver Transplant Recipients
Abstract
Background: The immunogenicity elicited by the Omicron BA.4/BA.5-adapted bivalent booster vaccine after solid organ transplantation (SOT) has not been characterized.
Methods: We assessed cell-mediated and neutralizing IgG antibody responses against the BA.4/BA.5 spike receptor-binding domain at baseline and 2 wk after the administration of an mRNA-based bivalent (ancestral strain and BA.4/BA.5 subvariants) vaccine among 30 SOT recipients who had received ≥3 monovalent vaccine doses. Previous coronavirus disease 2019 history was present in 46.7% of them. We also recruited a control group of 19 nontransplant healthy individuals. Cell-mediated immunity was measured by fluorescent ELISpot assay for interferon (IFN)-γ secretion, whereas the neutralizing IgG antibody response against the BA.4/BA.5 spike receptor-binding domain was quantified with a competitive ELISA.
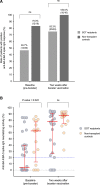
Results: The median number of BA.4/BA.5 spike-specific IFN-γ-producing spot-forming units (SFUs) increased from baseline to 2 wk postbooster (83.8 versus 133.0 SFUs/106 peripheral blood mononuclear cells; P = 0.0017). Seropositivity rate also increased (46.7%-83.3%; P = 0.001), as well as serum neutralizing activity (4.2%-78.3%; P < 0.0001). Patients with no prior coronavirus disease 2019 history experienced higher improvements in cell-mediated and neutralizing responses after booster vaccination. There was no correlation between BA.4/BA.5 spike-specific IFN-γ-producing SFUs and neutralizing activity. Nontransplant controls showed more robust postbooster cell-mediated immunity than SOT recipients (591.1 versus 133.0 IFN-γ-producing SFUs/106 peripheral blood mononuclear cells; P < 0.0001), although no differences were observed for antibody responses in terms of postbooster seropositivity rates or neutralizing activity.
Conclusions: Booster with the BA.4/BA.5-adapted bivalent vaccine generated strong subvariant-specific responses among SOT recipients. Booster-induced cell-mediated immunity, however, remained lower than in immunocompetent individuals.
Copyright © 2023 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Neutralizing antibody responses and cellular responses against SARS-CoV-2 Omicron subvariants after mRNA SARS-CoV-2 vaccination in kidney transplant recipients.Sci Rep. 2024 May 28;14(1):12176. doi: 10.1038/s41598-024-63147-z. Sci Rep. 2024. PMID: 38806644 Free PMC article.
-
Immunogenicity and safety of a bivalent (omicron BA.5 plus ancestral) SARS-CoV-2 recombinant spike protein vaccine as a heterologous booster dose: interim analysis of a phase 3, non-inferiority, randomised, clinical trial.Lancet Infect Dis. 2024 Jun;24(6):581-593. doi: 10.1016/S1473-3099(24)00077-X. Epub 2024 Mar 6. Lancet Infect Dis. 2024. PMID: 38460525 Clinical Trial.
-
Fourth dose bivalent COVID-19 vaccines outperform monovalent boosters in eliciting cross-reactive memory B cells to Omicron subvariants.J Infect. 2024 Oct;89(4):106246. doi: 10.1016/j.jinf.2024.106246. Epub 2024 Aug 8. J Infect. 2024. PMID: 39127451
-
Ancestral SARS-CoV-2 and Omicron BA.5-specific neutralizing antibody and T-cell responses after Omicron bivalent booster vaccination in previously infected and infection-naive individuals.J Med Virol. 2023 Aug;95(8):e28989. doi: 10.1002/jmv.28989. J Med Virol. 2023. PMID: 37565645
-
Neutralizing Antibody Response of the Wild-Type/Omicron BA.1 Bivalent Vaccine as the Second Booster Dose against Omicron BA.2 and BA.5.Microbiol Spectr. 2023 Mar 22;11(2):e0513122. doi: 10.1128/spectrum.05131-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36946738 Free PMC article.
Cited by
-
Torquetenovirus Loads in Peripheral Blood Predict Both the Humoral and Cell-Mediated Responses to SARS-CoV-2 Elicited by the mRNA Vaccine in Liver Transplant Recipients.Vaccines (Basel). 2023 Oct 28;11(11):1656. doi: 10.3390/vaccines11111656. Vaccines (Basel). 2023. PMID: 38005988 Free PMC article.
-
Neutralizing antibody responses and cellular responses against SARS-CoV-2 Omicron subvariants after mRNA SARS-CoV-2 vaccination in kidney transplant recipients.Sci Rep. 2024 May 28;14(1):12176. doi: 10.1038/s41598-024-63147-z. Sci Rep. 2024. PMID: 38806644 Free PMC article.
-
Comparison of immune responses to SARS-CoV-2 spike following Omicron infection or Omicron BA.4/5 vaccination in kidney transplant recipients.Front Immunol. 2025 Jan 14;15:1476294. doi: 10.3389/fimmu.2024.1476294. eCollection 2024. Front Immunol. 2025. PMID: 39877366 Free PMC article.
-
Antibody and T-Cell Response to Bivalent Booster SARS-CoV-2 Vaccines in People With Compromised Immune Function: COVERALL-3 Study.J Infect Dis. 2024 Oct 16;230(4):e847-e859. doi: 10.1093/infdis/jiae291. J Infect Dis. 2024. PMID: 38848312 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

