Faricimab: Transforming the Future of Macular Diseases Treatment - A Comprehensive Review of Clinical Studies
- PMID: 37746113
- PMCID: PMC10516184
- DOI: 10.2147/DDDT.S427416
Faricimab: Transforming the Future of Macular Diseases Treatment - A Comprehensive Review of Clinical Studies
Abstract
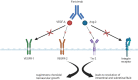
Degenerative eye conditions such as age-related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusion are major contributors to significant vision loss in developed nations. The primary therapeutic approach for managing complications linked to these diseases involves the intravitreal delivery of anti-vascular endothelial growth factor (VEGF) treatments. Faricimab is a novel, humanised, bispecific antibody that simultaneously binds all VEGF-A isoforms and Angiopoietin-2, which has been approved by regulatory agencies, such as the US Food and Drug Administration (FDA), the UK Medicines and Healthcare products Regulatory Agency (MHRA) and the European Medicines Agency (EMA), for the treatment of neovascular AMD and diabetic macular oedema (DMO). Intravitreal faricimab holds the promise of reducing the treatment burden for patients with these conditions by achieving comparable or superior therapeutic outcomes with fewer clinic visits. The scope of faricimab's application includes addressing complex macular conditions such as DMO. This review intends to elucidate the distinctive pharmacological characteristics of faricimab and provide an overview of the key clinical trials and real-world studies that assess its effectiveness and safety in treating degenerative macular diseases.
Keywords: age-related macular degeneration; anti-VEGF; degenerative macular disorders; diabetic macular oedema; diabetic retinopathy; efficacy; faricimab; intravitreal treatment; retinal vein occlusion; safety.
© 2023 Panos et al.
Conflict of interest statement
Winfried M Amoaku has provided consultancy services to Alcon, Alimera, Allergan (AbbVie), Bayer, Novartis and Roche. The other authors report no conflicts of interest in this work.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials