Multiple Fungi May Connect the Roots of an Orchid (Cypripedium reginae) and Ash (Fraxinus nigra) in Western Newfoundland
- PMID: 37746191
- PMCID: PMC10512338
- DOI: 10.3389/ffunb.2022.805127
Multiple Fungi May Connect the Roots of an Orchid (Cypripedium reginae) and Ash (Fraxinus nigra) in Western Newfoundland
Abstract
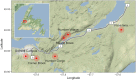
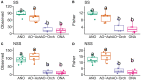
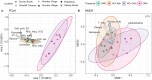
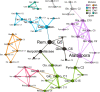
Showy lady's slipper (Cypripedium reginae Walter, Orchidaceae) and black ash (Fraxinus nigra Marshall, Oleaceae) often co-occur in close proximity in fens in western Newfoundland, Canada. Metabarcoding of DNA extracted from root samples of both species following surface sterilization, and others without surface sterilization was used to determine if there were shared fungal endophytes in the roots of both species that could form a common mycorrhizal network between them. A wide variety of fungi were recovered from primers amplifying the nuclear ribosomal internal transcribed spacer region (ITS2). Sixty-six fungal sequences were shared by surface-sterilized roots of both orchid and ash, among them arbuscular mycorrhizal fungi (Claroideoglomus, Dominikia, Glomus and Rhizophagus), ectomycorrhizal fungi (Inocybe and Tomentella), the broad-host root endophyte Cadophora orchidicola, along with root pathogens (Dactylonectria, Ilyonectria, Pyricularia, and Xylomyces) and fungi of unknown function. There appear to be multiple fungi that could form a common mycorrhizal network between C. reginae and F. nigra, which might explain their frequent co-occurrence. Transfer of nutrients or carbon between the orchid and ash via one or more of the shared fungal endophytes remains to be demonstrated.
Keywords: ITS2; Illumina MiSeq; black ash; common mycorrhizal network; metabarcoding; mixotrophy; mycorrhiza; showy lady's slipper orchid.
Copyright © 2022 Weerasuriya, Kukolj, Spencer, Sveshnikov and Thorn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
High specificity generally characterizes mycorrhizal association in rare lady's slipper orchids, genus Cypripedium.Mol Ecol. 2005 Feb;14(2):613-26. doi: 10.1111/j.1365-294X.2005.02424.x. Mol Ecol. 2005. PMID: 15660950
-
Exploration of Mycobiota in Cypripedium japonicum, an Endangered Species.Mycobiology. 2022 Apr 29;50(2):142-149. doi: 10.1080/12298093.2022.2064409. eCollection 2022. Mycobiology. 2022. PMID: 35571859 Free PMC article.
-
The evolutionary history of mycorrhizal specificity among lady's slipper orchids.Evolution. 2007 Jun;61(6):1380-90. doi: 10.1111/j.1558-5646.2007.00112.x. Evolution. 2007. PMID: 17542847
-
Further advances in orchid mycorrhizal research.Mycorrhiza. 2007 Sep;17(6):475-486. doi: 10.1007/s00572-007-0138-1. Epub 2007 Jun 21. Mycorrhiza. 2007. PMID: 17582535 Review.
-
Mycorrhizal fungi affect orchid distribution and population dynamics.New Phytol. 2018 Sep;219(4):1207-1215. doi: 10.1111/nph.15223. Epub 2018 May 23. New Phytol. 2018. PMID: 29790578 Review.
Cited by
-
Diversity of unique, nonmycorrhizal endophytic fungi in cultivated Phalaenopsis orchids: A pilot study.Plant Environ Interact. 2024 May 17;5(3):e10146. doi: 10.1002/pei3.10146. eCollection 2024 Jun. Plant Environ Interact. 2024. PMID: 38764601 Free PMC article.
References
-
- Abarenkov K., Zirk A., Piirmann T., Pöhönen R., Ivanov F., Nilsson R. H., et al. . (2021). UNITE General FASTA Release for Fungi. Version 10.05.2021. UNITE Community.
-
- An J., Liu C., Wang Q., Yao M., Rui J., Zhang S., et al. . (2019). Soil bacterial community structure in Chinese wetlands. Geoderma 337, 290–299. 10.1016/j.geoderma.2018.09.035 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

