Evaluation of an AAV9-mini-dystrophin gene therapy candidate in a rat model of Duchenne muscular dystrophy
- PMID: 37746247
- PMCID: PMC10512999
- DOI: 10.1016/j.omtm.2023.05.017
Evaluation of an AAV9-mini-dystrophin gene therapy candidate in a rat model of Duchenne muscular dystrophy
Abstract
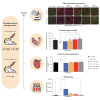
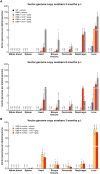
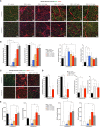
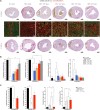
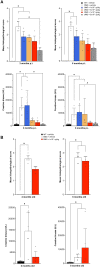
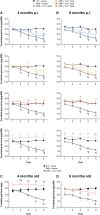
Duchenne muscular dystrophy (DMD) is an X-linked disease caused by loss-of-function mutations in the dystrophin gene and is characterized by muscle wasting and early mortality. Adeno-associated virus-mediated gene therapy is being investigated as a treatment for DMD. In the nonclinical study documented here, we determined the effective dose of fordadistrogene movaparvovec, a clinical candidate adeno-associated virus serotype 9 vector carrying a human mini-dystrophin transgene, after single intravenous injection in a dystrophin-deficient (DMDmdx) rat model of DMD. Overall, we found that transduction efficiency, number of muscle fibers expressing the human mini-dystrophin polypeptide, improvement of the skeletal and cardiac muscle tissue architecture, correction of muscle strength and fatigability, and improvement of diastolic and systolic cardiac function were directly correlated with the amount of vector administered. The effective dose was then tested in older DMDmdx rats with a more dystrophic phenotype similar to the pathology observed in older patients with DMD. Except for a less complete rescue of muscle function in the oldest cohort, fordadistrogene movaparvovec was also found to be therapeutically effective in older DMDmdx rats, suggesting that this product may be appropriate for evaluation in patients with DMD at all stages of disease.
Keywords: DMDmdx rat; Duchenne muscular dystrophy; age; dose study; gene therapy; mini-dystrophin; recombinant adeno-associated virus.
© 2023 The Authors.
Figures

Similar articles
-
Dystrophin/mini-dystrophin expression analysis by immunoaffinity liquid chromatography-tandem mass spectrometry after gene therapy for DMD.Gene Ther. 2025 Aug 2. doi: 10.1038/s41434-025-00554-5. Online ahead of print. Gene Ther. 2025. PMID: 40753271
-
Long-Term Protective Effect of Human Dystrophin Expressing Chimeric (DEC) Cell Therapy on Amelioration of Function of Cardiac, Respiratory and Skeletal Muscles in Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2022 Dec;18(8):2872-2892. doi: 10.1007/s12015-022-10384-2. Epub 2022 May 19. Stem Cell Rev Rep. 2022. PMID: 35590083 Free PMC article.
-
Dose-Escalation Study of Systemically Delivered rAAVrh74.MHCK7.micro-dystrophin in the mdx Mouse Model of Duchenne Muscular Dystrophy.Hum Gene Ther. 2021 Apr;32(7-8):375-389. doi: 10.1089/hum.2019.255. Epub 2021 Feb 18. Hum Gene Ther. 2021. PMID: 33397205 Free PMC article. Clinical Trial.
-
[Gene therapy for muscular dystrophy].No To Hattatsu. 2004 Mar;36(2):117-23. No To Hattatsu. 2004. PMID: 15031985 Review. Japanese.
-
Gene therapy in Duchenne muscular dystrophy.Brain Dev. 1996 Sep-Oct;18(5):357-61. doi: 10.1016/0387-7604(96)00043-5. Brain Dev. 1996. PMID: 8891229 Review.
Cited by
-
Long-Term Dystrophin Replacement Therapy in Duchenne Muscular Dystrophy Causes Cardiac Inflammation.JACC Basic Transl Sci. 2025 Jun;10(6):759-782. doi: 10.1016/j.jacbts.2024.12.015. Epub 2025 Mar 12. JACC Basic Transl Sci. 2025. PMID: 40562489 Free PMC article.
-
The road toward AAV-mediated gene therapy of Duchenne muscular dystrophy.Mol Ther. 2025 May 7;33(5):2035-2051. doi: 10.1016/j.ymthe.2025.03.065. Epub 2025 Apr 2. Mol Ther. 2025. PMID: 40181545 Review.
-
Dystrophin/mini-dystrophin expression analysis by immunoaffinity liquid chromatography-tandem mass spectrometry after gene therapy for DMD.Gene Ther. 2025 Aug 2. doi: 10.1038/s41434-025-00554-5. Online ahead of print. Gene Ther. 2025. PMID: 40753271
-
AAV mini-dystrophin gene therapy for Duchenne muscular dystrophy: a phase 1b trial.Nat Med. 2025 Aug;31(8):2712-2721. doi: 10.1038/s41591-025-03750-3. Epub 2025 Jun 27. Nat Med. 2025. PMID: 40579547 Free PMC article.
-
Swine reporter model for preclinical evaluation and characterization of gene delivery vectors.bioRxiv [Preprint]. 2025 Jun 14:2025.06.13.659546. doi: 10.1101/2025.06.13.659546. bioRxiv. 2025. PMID: 40661506 Free PMC article. Preprint.
References
LinkOut - more resources
Full Text Sources

