Systemic gene therapy using an AAV44.9 vector rescues a neonatal lethal mouse model of propionic acidemia
- PMID: 37746248
- PMCID: PMC10512014
- DOI: 10.1016/j.omtm.2023.06.008
Systemic gene therapy using an AAV44.9 vector rescues a neonatal lethal mouse model of propionic acidemia
Abstract
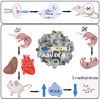
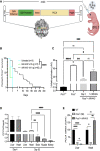
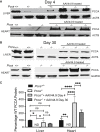
Propionic acidemia (PA) is rare autosomal recessive metabolic disorder caused by defects in the mitochondrially localized enzyme propionyl-coenzyme A (CoA) carboxylase. Patients with PA can suffer from lethal metabolic decompensation and cardiomyopathy despite current medical management, which has led to the pursuit of gene therapy as a new treatment option for patients. Here we assess the therapeutic efficacy of a recently described adeno-associated virus (AAV) capsid, AAV44.9, to deliver a therapeutic PCCA transgene in a new mouse model of propionyl-CoA carboxylase α (PCCA) deficiency generated by genome editing. Pcca-/- mice recapitulate the severe neonatal presentation of PA and manifest uniform neonatal lethality, absent PCCA expression, and increased 2-methylcitrate. A single injection of the AAV44.9 PCCA vector in the immediate newborn period, systemically delivered at a dose of 1e11 vector genome (vg)/pup but not 1e10 vg/pup, increased survival, reduced plasma methylcitrate, and resulted in high levels of transgene expression in the liver and heart in treated Pcca-/- mice. Our studies not only establish a versatile and accurate new mouse model of PA but further demonstrate that the AAV44.9 vectors may be suitable for treatment of many metabolic disorders where hepato-cardiac transduction following systemic delivery is desired, such as PA, and, by extension, fatty acid oxidation defects and glycogen storage disorders.
Keywords: AAV44.9; PCCA; cardiac tropism; genome editing; mouse model; neonatal gene therapy; organic acidemia; propionic acidemia; propionylcoa carboxylase deficiency; systemic gene therapy.
Conflict of interest statement
R.J.C., G.D.P., J.A.C., and C.P.V. are inventors on a patent application filed by the NIH on their behalf on use of AAV44.9 as a gene therapy vector to treat methylmalonic acidemia.
Figures


References
-
- Shchelochkov O.A., Carrillo N., Venditti C. In: GeneReviews((R)) Adam M.P., Everman D.B., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Amemiya A., editors. 1993. Propionic Acidemia.
Grants and funding
LinkOut - more resources
Full Text Sources

